Xenogeneic-free defined conditions for derivation and expansion of human embryonic stem cells with mesenchymal stem cells
- PMID: 31245438
- PMCID: PMC6581821
- DOI: 10.1016/j.reth.2014.12.004
Xenogeneic-free defined conditions for derivation and expansion of human embryonic stem cells with mesenchymal stem cells
Abstract
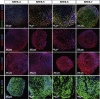
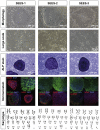
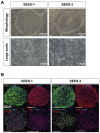
The potential applications of human embryonic stem cells (hESCs) in regenerative medicine and developmental research have made stem cell biology one of the most fascinating and rapidly expanding fields of biomedicine. The first clinical trial of hESCs in humans has begun, and the field of stem cell therapy has just entered a new era. Here, we report seven hESC lines (SEES-1, -2, -3, -4, -5, -6, and -7). Four of them were derived and maintained on irradiated human mesenchymal stem cells (hMSCs) grown in xenogeneic-free defined media and substrate. Xenogeneic-free hMSCs isolated from the subcutaneous tissue of extra fingers from individuals with polydactyly showed appropriate potentials as feeder layers in the pluripotency and growth of hESCs. In this report, we describe a comprehensive characterization of these newly derived SEES cell lines. In addition, we developed a scalable culture system for hESCs having high biological safety by using gamma-irradiated serum replacement and pharmaceutical-grade recombinant basic fibroblast growth factor (bFGF, also known as trafermin). This is first report describing the maintenance of hESC pluripotency using pharmaceutical-grade human recombinant bFGF (trafermin) and gamma-irradiated serum replacement. Our defined medium system provides a path to scalability in Good Manufacturing Practice (GMP) settings for the generation of clinically relevant cell types from pluripotent cells for therapeutic applications.
Keywords: Gamma irradiation; Human embryonic stem cells; Human feeder layer; Mesenchymal stem cells; Stem cell expansion; Xenogeneic-free medium.
Figures












Similar articles
-
Feeder-free maintenance of hESCs in mesenchymal stem cell-conditioned media: distinct requirements for TGF-beta and IGF-II.Cell Res. 2009 Jun;19(6):698-709. doi: 10.1038/cr.2009.35. Cell Res. 2009. PMID: 19308090
-
Maintenance of human embryonic stem cells in media conditioned by human mesenchymal stem cells obviates the requirement of exogenous basic fibroblast growth factor supplementation.Tissue Eng Part C Methods. 2012 May;18(5):387-96. doi: 10.1089/ten.TEC.2011.0546. Epub 2012 Jan 26. Tissue Eng Part C Methods. 2012. PMID: 22136131
-
Feeder & basic fibroblast growth factor-free culture of human embryonic stem cells: Role of conditioned medium from immortalized human feeders.Indian J Med Res. 2016 Dec;144(6):838-851. doi: 10.4103/ijmr.IJMR_424_15. Indian J Med Res. 2016. PMID: 28474621 Free PMC article.
-
Identification and characterization of small-molecule ligands that maintain pluripotency of human embryonic stem cells.Biochem Soc Trans. 2010 Aug;38(4):1058-61. doi: 10.1042/BST0381058. Biochem Soc Trans. 2010. PMID: 20659003 Review.
-
Human embryonic stem cell cultivation: historical perspective and evolution of xeno-free culture systems.Reprod Biol Endocrinol. 2015 Feb 22;13:9. doi: 10.1186/s12958-015-0005-4. Reprod Biol Endocrinol. 2015. PMID: 25890180 Free PMC article. Review.
Cited by
-
Genes Involved in DNA Repair and Mitophagy Protect Embryoid Bodies from the Toxic Effect of Methylmercury Chloride under Physioxia Conditions.Cells. 2023 Jan 21;12(3):390. doi: 10.3390/cells12030390. Cells. 2023. PMID: 36766732 Free PMC article.
-
Organoids recapitulate organs?Stem Cell Investig. 2018 Jan 17;5:3. doi: 10.21037/sci.2018.01.02. eCollection 2018. Stem Cell Investig. 2018. PMID: 29430459 Free PMC article. No abstract available.
-
Establishment of a rapid and footprint-free protocol for differentiation of human embryonic stem cells into pancreatic endocrine cells with synthetic mRNAs encoding transcription factors.Stem Cell Res Ther. 2018 Oct 25;9(1):277. doi: 10.1186/s13287-018-1038-3. Stem Cell Res Ther. 2018. PMID: 30359326 Free PMC article.
-
Gorlin syndrome-induced pluripotent stem cells form medulloblastoma with loss of heterozygosity in PTCH1.Aging (Albany NY). 2020 May 21;12(10):9935-9947. doi: 10.18632/aging.103258. Epub 2020 May 21. Aging (Albany NY). 2020. PMID: 32436863 Free PMC article.
-
Automated xeno-free chondrogenic differentiation from human embryonic stem cells: Enhancing efficiency and ensuring high-quality mass production.Regen Ther. 2024 Sep 10;26:889-900. doi: 10.1016/j.reth.2024.09.007. eCollection 2024 Jun. Regen Ther. 2024. PMID: 39822341 Free PMC article.
References
-
- Thomson J.A., Itskovitz-Eldor J., Shapiro S.S., Waknitz M.A., Swiergiel J.J., Marshall V.S. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. - PubMed
-
- Amit M., Carpenter M.K., Inokuma M.S., Chiu C.P., Harris C.P., Waknitz M.A. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev Biol. 2000;227:271–278. - PubMed
-
- Cowan C.A., Klimanskaya I., McMahon J., Atienza J., Witmyer J., Zucker J.P. Derivation of embryonic stem-cell lines from human blastocysts. N Engl J Med. 2004;350:1353–1356. - PubMed
References in supplemental figures
-
- Hosoi E. Biological and clinical aspects of ABO blood group system. J Med Invest. 2008;55:174–182. (For Supplemental Figure S1) - PubMed
-
- Ota M., Fukushima H., Kulski J.K., Inoko H. Single nucleotide polymorphism detection by polymerase chain reaction-restriction fragment length polymorphism. Nat Protoc. 2007;2:2857–2864. (For Supplemental Figure S2) - PubMed
-
- Muro T., Fujihara J., Imamura S., Nakamura H., Kimura-Kataoka K., Toga T. Determination of ABO genotypes by real-time PCR using allele-specific primers. Leg Med Tokyo. 2012;14:47–50. (For Supplemental Figure S3) - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

