Characterization of in vivo tumorigenicity tests using severe immunodeficient NOD/Shi-scid IL2Rγnull mice for detection of tumorigenic cellular impurities in human cell-processed therapeutic products
- PMID: 31245439
- PMCID: PMC6581766
- DOI: 10.1016/j.reth.2014.12.001
Characterization of in vivo tumorigenicity tests using severe immunodeficient NOD/Shi-scid IL2Rγnull mice for detection of tumorigenic cellular impurities in human cell-processed therapeutic products
Abstract
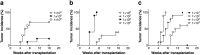
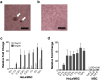
The contamination of human cell-processed therapeutic products (hCTPs) with tumorigenic cells is one of the major concerns in the manufacturing and quality control of hCTPs. However, no quantitative method for detecting the tumorigenic cellular impurities is currently standardized. NOD/Shi-scid IL2Rγnull (NOG) mice have shown high xeno-engraftment potential compared with other well-known immunodeficient strains, e.g. nude mice. Hypothesizing that tumorigenicity test using NOG mice could be a sensitive and quantitative method to detect a small amount of tumorigenic cells in hCTPs, we examined tumor formation after subcutaneous transplantation of HeLa cells, as a model of tumorigenic cells, in NOG mice and nude mice. Sixteen weeks after inoculation, the 50% tumor-producing dose (TPD50) values of HeLa cells were stable at 1.3 × 104 and 4.0 × 105 cells in NOG and nude mice, respectively, indicating a 30-fold higher sensitivity of NOG mice compared to that of nude mice. Transplanting HeLa cells embedded with Matrigel in NOG mice further decreased the TPD50 value to 7.9 × 10 cells, leading to a 5000-fold higher sensitivity, compared with that of nude mice. Additionally, when HeLa cells were mixed with 106 or 107 human mesenchymal stem cells as well as Matrigel, the TPD50 values in NOG mice were comparable to those of HeLa cells alone with Matrigel. These results suggest that the in vivo tumorigenicity test using NOG mice with Matrigel is a highly sensitive and quantitative method to detect a trace amount of tumorigenic cellular impurities in human somatic cells, which can be useful in the quality assessment of hCTPs.
Keywords: Cellular therapy; NOG mice; Quality control; Regenerative medicine; Tumorigenicity test.
Figures



References
-
- World Health Organization . 1998. Requirements for the use of animal cells as in vitro substrates for the production of biologicals. (WHO technical report series). No 878 Annex 1.
-
- Kuroda T., Yasuda S., Sato Y. Tumorigenicity studies for human pluripotent stem cell-derived products. Biological Pharm Bull. 2013;36(2):189–192. - PubMed
-
- Goldman J.P., Blundell M.P., Lopes L., Kinnon C., Di Santo J.P., Thrasher A.J. Enhanced human cell engraftment in mice deficient in RAG2 and the common cytokine receptor gamma chain. Br J Haematol. 1998;103(2):335–342. - PubMed
-
- Ito M., Hiramatsu H., Kobayashi K., Suzue K., Kawahata M., Hioki K. NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002;100(9):3175–3182. - PubMed
-
- Shultz L.D., Lyons B.L., Burzenski L.M., Gott B., Chen X., Chaleff S. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol. 2005;174(10):6477–6489. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

