A Carbon-Fiber Sheet Resistor for MR-, CT-, SPECT-, and PET-Compatible Temperature Maintenance in Small Animals
- PMID: 31245549
- PMCID: PMC6588203
- DOI: 10.18383/j.tom.2019.00008
A Carbon-Fiber Sheet Resistor for MR-, CT-, SPECT-, and PET-Compatible Temperature Maintenance in Small Animals
Abstract
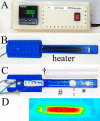
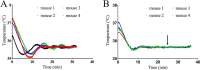
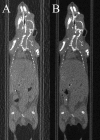
A magnetic resonance (MR)-, computed tomography (CT)-, single-photon emission computed tomography (SPECT)-, and positron emission tomography (PET)-compatible carbon-fiber sheet resistor for temperature maintenance in small animals where space limitations prevent the use of circulating fluids was developed. A 250 Ω carbon-fiber sheet resistor was mounted to the underside of an imaging cradle. Alternating current, operating at 99 kHz, and with a power of 1-2 W, was applied to the resistor providing a cradle base temperature of ∼37°C. Temperature control was implemented with a proportional-integral-derivative controller, and temperature maintenance was demonstrated in 4 mice positioned in both MR and PET/SPECT/CT scanners. MR and CT compatibility were also shown, and multimodal MR-CT-PET-SPECT imaging of the mouse abdomen was performed in vivo. Core temperature was maintained at 35.5°C ± 0.2°C. No line-shape, frequency, or image distortions attributable to the current flow through the heater were observed on MR. Upon CT imaging, no heater-related artifacts were observed when carbon-fiber was used. Multimodal imaging was performed and images could be easily coregistered, displayed, analyzed, and presented. Carbon fiber sheet resistors powered with high-frequency alternating current allow homeothermic maintenance that is compatible with multimodal imaging. The heater is small, and it is easy to produce and integrate into multimodal imaging cradles.
Keywords: MR-CT-PET-SPECT compatibility; carbon fiber; heater; multimodal imaging.
Conflict of interest statement
Conflict of Interest: The authors have no conflict of interest to declare.
Figures
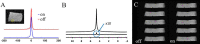

Similar articles
-
PET/MRI with diagnostic MR sequences vs PET/CT in the detection of abdominal and pelvic cancer.Eur J Radiol. 2016 Apr;85(4):751-9. doi: 10.1016/j.ejrad.2016.01.010. Epub 2016 Jan 21. Eur J Radiol. 2016. PMID: 26971419
-
A resistive heating system for homeothermic maintenance in small animals.Magn Reson Imaging. 2015 Jul;33(6):847-51. doi: 10.1016/j.mri.2015.03.011. Epub 2015 Apr 8. Magn Reson Imaging. 2015. PMID: 25863135 Free PMC article.
-
Evaluating the Diagnostic Efficacy of 99mTc-Methionine Single-Photon Emission Computed Tomography-Computed Tomography: A Head-to-Head Comparison with 11C-Methionine Positron Emission Tomography-Magnetic Resonance Imaging in Glioma Patients.Cancer Biother Radiopharm. 2024 Jun;39(5):349-357. doi: 10.1089/cbr.2023.0147. Epub 2024 Feb 7. Cancer Biother Radiopharm. 2024. PMID: 38324045
-
PET-MR and SPECT-MR multimodality probes: Development and challenges.Theranostics. 2018 Nov 29;8(22):6210-6232. doi: 10.7150/thno.26610. eCollection 2018. Theranostics. 2018. PMID: 30613293 Free PMC article. Review.
-
Morphology supporting function: attenuation correction for SPECT/CT, PET/CT, and PET/MR imaging.Q J Nucl Med Mol Imaging. 2016 Mar;60(1):25-39. Epub 2015 Nov 17. Q J Nucl Med Mol Imaging. 2016. PMID: 26576737 Free PMC article. Review.
Cited by
-
Ultrasound-Mediated Gemcitabine Delivery Reduces the Normal-Tissue Toxicity of Chemoradiation Therapy in a Muscle-Invasive Bladder Cancer Model.Int J Radiat Oncol Biol Phys. 2021 Apr 1;109(5):1472-1482. doi: 10.1016/j.ijrobp.2020.11.046. Int J Radiat Oncol Biol Phys. 2021. PMID: 33714528 Free PMC article.
-
Manganese-free chow, a refined non-invasive solution to reduce gastrointestinal signal for T1-weighted magnetic resonance imaging of the mouse abdomen.Lab Anim. 2020 Aug;54(4):353-364. doi: 10.1177/0023677219869363. Epub 2019 Sep 16. Lab Anim. 2020. PMID: 31526094 Free PMC article.
-
A System-Agnostic, Adaptable and Extensible Animal Support Cradle System for Cardio-Respiratory-Synchronised, and Other, Multi-Modal Imaging of Small Animals.Tomography. 2021 Feb 7;7(1):39-54. doi: 10.3390/tomography7010004. eCollection 2021 Mar. Tomography. 2021. PMID: 33681462 Free PMC article.
-
Minimally invasive measurement of carotid artery and brain temperature in the mouse.Magn Reson Med. 2025 May;93(5):2049-2058. doi: 10.1002/mrm.30405. Epub 2025 Jan 8. Magn Reson Med. 2025. PMID: 39775951 Free PMC article.
-
Imaging angiogenesis in an intracerebrally induced model of brain macrometastasis using αv β3 -targeted iron oxide microparticles.NMR Biomed. 2023 Apr 10;36(9):e4948. doi: 10.1002/nbm.4948. Online ahead of print. NMR Biomed. 2023. PMID: 37038086 Free PMC article.
References
-
- Kersemans V, Beech JS, Gilchrist S, Kinchesh P, Allen PD, Thompson J, Gomes AL, D'Costa Z, Bird L, Tullis IDC, Newman RG, Corroyer-Dulmont A, Falzone N, Azad A, Vallis KA, Sansom OJ, Muschel RJ, Vojnovic B, Hill MA, Fokas E, Smart SC. An efficient and robust MRI-guided radiotherapy planning approach for targeting abdominal organs and tumours in the mouse. PLoS One. 2017;12:e0176693. - PMC - PubMed
-
- Torossian A, Ruehlmann S, Middeke M, Sessler DI, Lorenz W, Wulf HF, Bauhofer A. Mild preseptic hypothermia is detrimental in rats. Crit Care Med. 2004;32:1899–1903. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

