Coumarins from the peel of citrus grown in Colombia: composition, elicitation and antifungal activity
- PMID: 31245648
- PMCID: PMC6582165
- DOI: 10.1016/j.heliyon.2019.e01937
Coumarins from the peel of citrus grown in Colombia: composition, elicitation and antifungal activity
Abstract
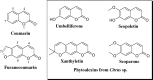
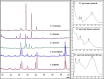
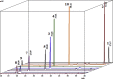
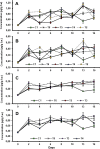
The present work analyses the chromatographic profile of the peels from fruits of different citrus cultivated in Colombia: sweet orange (Citrus sinensis [L.] Osbeck var. Valencia), mandarins (Citrus reticulata L. var. Arrayana and Oneco), Key lime (Citrus aurantifolia [Christ.] Swingle var. Pajarito), Mandarine lime (Citrus x limonia, a hybrid between Citrus reticulata and Citrus x limon) and Tahitian lime (C. latifolia Tanaka, syn. Persian lime). Coumarins, furanocoumarins and polymethoxylated flavones are the major compounds. Then, six coumarins were isolated and identified from fruits of Tahitian and Key lime corresponding to 5-geranyloxy-7-methoxycoumarin; 5,7-dimethoxycoumarin (syn. limettin); 5,8-dimethoxypsoralen (syn. isopimpinellin); 5-methoxypsoralen (syn. bergaptene); 5-geranoxypsoralen (syn. bergamottin) and 5-(2,3-dihydroxy-3-methylbutoxy) psoralen (syn. oxypeucedanin hydrate). Coumarins and furanocoumarins were quantified by liquid chromatography (HPLC-DAD). Results show that the prenylated compounds were present in high concentrations in Tahitian and Key lime but in very low amounts in mandarins and sweet orange. Subsequently, the antifungal activity (inhibition of mycelial growth and germination of spores) of the coumarins against the fungus causing the anthracnose, Colletotrichum sp. (isolated from aerial parts of Tahitian lime) was determined. The compounds limettin and bergaptene, as well as mixtures of them, showed significant inhibitory effect (radial growth and spore germination) when compared to the control. Finally, the effect of some recognized elicitors to induce the coumarin production in fruits of C. latifolia was evaluated. The results showed that the chemical profiles are dependent on the applied elicitor and the post-induction time. As a result of the induction, a high concentration of some coumarins and furanocoumarins was maintained in the course of time for the Tahitian lime. In conclusion, isolated coumarins could be involved in the defense mechanisms of C. latifolia, C. aurantifolia and C. limonia and their accumulation may be modulated by the application of elicitors.
Keywords: Antifungal activity; Citrus fruits; Coumarin; Elicitors; Food science; Furanocoumarins; Limettin; Plant elicitor molecule; Rutaceae; Spore germination inhibition; Tahitian lime.
Figures



Similar articles
-
Coumarins, psoralens, and quantitative 1H-NMR spectroscopy for authentication of lemon (Citrus limon [L.] Burm.f.) and Persian lime (Citrus × latifolia [Yu.Tanaka] Tanaka) juices.Food Chem. 2021 Oct 15;359:129804. doi: 10.1016/j.foodchem.2021.129804. Epub 2021 Apr 19. Food Chem. 2021. PMID: 34015560
-
Metabolomic Investigation of Citrus latifolia and the Putative Role of Coumarins in Resistance to Black Spot Disease.Front Mol Biosci. 2022 Jun 24;9:934401. doi: 10.3389/fmolb.2022.934401. eCollection 2022. Front Mol Biosci. 2022. PMID: 35813812 Free PMC article.
-
Phototoxic coumarins in limes.Food Chem Toxicol. 1993 May;31(5):331-5. doi: 10.1016/0278-6915(93)90187-4. Food Chem Toxicol. 1993. PMID: 8505017
-
Biological Activities and Safety of Citrus spp. Essential Oils.Int J Mol Sci. 2018 Jul 5;19(7):1966. doi: 10.3390/ijms19071966. Int J Mol Sci. 2018. PMID: 29976894 Free PMC article. Review.
-
Volatile Compounds in Citrus Essential Oils: A Comprehensive Review.Front Plant Sci. 2019 Feb 5;10:12. doi: 10.3389/fpls.2019.00012. eCollection 2019. Front Plant Sci. 2019. PMID: 30804951 Free PMC article. Review.
Cited by
-
Mechanistic Insights into the Ameliorating Effect of Melanogenesis of Psoralen Derivatives in B16F10 Melanoma Cells.Molecules. 2022 Apr 19;27(9):2613. doi: 10.3390/molecules27092613. Molecules. 2022. PMID: 35565964 Free PMC article.
-
Safety and efficacy of a feed additive consisting of an essential oil obtained from the fruit of Apium graveolens L. (celery seed oil) for all animal species (FEFANA asbl).EFSA J. 2024 Jul 25;22(7):e8907. doi: 10.2903/j.efsa.2024.8907. eCollection 2024 Jul. EFSA J. 2024. PMID: 39055667 Free PMC article.
-
Citrus × Clementina Hort. Juice Enriched with Its By-Products (Peels and Leaves): Chemical Composition, In Vitro Bioactivity, and Impact of Processing.Antioxidants (Basel). 2020 Apr 3;9(4):298. doi: 10.3390/antiox9040298. Antioxidants (Basel). 2020. PMID: 32260119 Free PMC article.
-
Ethnobotany and diversity of Citrus spp. (Rutaceae) as a source of "Kem-kem" traditional medicine used among the Karo sub-ethnic in North Sumatra, Indonesia.Heliyon. 2024 Apr 16;10(9):e29721. doi: 10.1016/j.heliyon.2024.e29721. eCollection 2024 May 15. Heliyon. 2024. PMID: 38694125 Free PMC article.
-
Characterisation, pathogenicity and hydrolytic enzyme profiling of selected Fusarium species and their inhibition by novel coumarins.Arch Microbiol. 2021 Aug;203(6):3495-3508. doi: 10.1007/s00203-021-02335-1. Epub 2021 Apr 28. Arch Microbiol. 2021. PMID: 33912984
References
-
- Afek U., Sztejnberg A. Scoparone (6,7- dimethoxycoumarin), a citrus phytoalexin involved in resistance to pathogens. In: Dniel M., Purkayastha R.P., editors. Handbook of Phytoalexin Metabolismand Action. Marcel Dekker Inc.; New York, Basel, Hong Kong: 1994. pp. 262–286.
-
- Afek U., Sztejnberg A. Accumulation of scoparone, a phytoalexin associated with resistance of citrus to Phytophthora citrophthora. Phytopathology. 1988;78:1678–1682.
-
- Afek U., Orenstein J., Carmeli S., Rodov V., Joseph M.B. Umbelliferone: a phytoalexin associated with resistance of immature Marsh grapefruit to Penicillium digitatum. Phytochemistry. 1999;50:1129–1132.
-
- Ahuja I., Kissen R., Bones A.M. Phytoalexins in defense against pathogens. Trends Plant Sci. 2012;17:73–90. - PubMed
-
- Araújo R.S.A., Guerra F.Q.S., Lima E.O., de Simone C.A., Tavares J.F., Scotti L., Scotti M.T., de Aquino T.M., de Moura R.O., Mendonça F.J.B., Barbosa-Filho J.M. Synthesis, structure-activity relationships (SAR) and in silico studies of coumarin derivatives with antifungal activity. Int. J. Mol. Sci. 2013;14:1293–1309. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

