Comparison of alfalfa plants overexpressing glutamine synthetase with those overexpressing sucrose phosphate synthase demonstrates a signaling mechanism integrating carbon and nitrogen metabolism between the leaves and nodules
- PMID: 31245757
- PMCID: PMC6508842
- DOI: 10.1002/pld3.115
Comparison of alfalfa plants overexpressing glutamine synthetase with those overexpressing sucrose phosphate synthase demonstrates a signaling mechanism integrating carbon and nitrogen metabolism between the leaves and nodules
Abstract
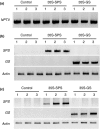
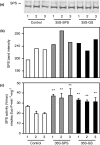
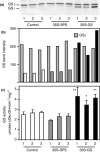
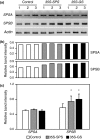
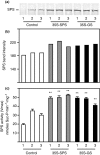
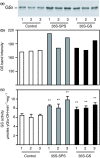
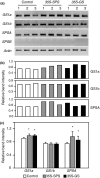
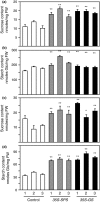
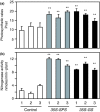
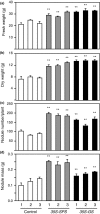
Alfalfa, like other legumes, establishes a symbiotic relationship with the soil bacteria, Sinorhizobium meliloti, which results in the formation of the root nodules. Nodules contain the bacteria enclosed in a membrane-bound vesicle, the symbiosome where it fixes atmospheric N2 and converts it into ammonia using the bacterial enzyme, nitrogenase. The ammonia released into the cytoplasm from the symbiosome is assimilated into glutamine (Gln) using carbon skeletons produced by the metabolism of sucrose (Suc), which is imported into the nodules from the leaves. The key enzyme involved in the synthesis of Suc in the leaves is sucrose phosphate synthase (SPS) and glutamine synthetase (GS) is the enzyme with a role in ammonia assimilation in the root nodules. Alfalfa plants, overexpressing SPS or GS, or both showed increased growth and an increase in nodule function. The endogenous genes for the key enzymes in C/N metabolism showed increased expression in the nodules of both sets of transformants. Furthermore, the endogenous SPS and GS genes were also induced in the leaves and nodules of the transformants, irrespective of the transgene, suggesting that the two classes of plants share a common signaling pathway regulating C/N metabolism in the nodules. This study reaffirms the utility of the nodulated legume plant to study C/N interaction and the cross talk between the source and sink for C and N.
Keywords: SPS and GS activity; forage quality; malate dehydrogenase; nitrogenase activity; phosphoenolpyruvate carboxylase; sucrose synthase.
Conflict of interest statement
The authors declare no conflict of interest associated with the work described in this manuscript.
Figures


Similar articles
-
Distinct nodule and leaf functions of two different sucrose phosphate synthases in alfalfa.Planta. 2019 Nov;250(5):1743-1755. doi: 10.1007/s00425-019-03261-9. Epub 2019 Aug 17. Planta. 2019. PMID: 31422508
-
Nodule-enhanced expression of a sucrose phosphate synthase gene member (MsSPSA) has a role in carbon and nitrogen metabolism in the nodules of alfalfa (Medicago sativa L.).Planta. 2010 Jan;231(2):233-44. doi: 10.1007/s00425-009-1043-y. Epub 2009 Nov 8. Planta. 2010. PMID: 19898977 Free PMC article.
-
Transgenic alfalfa (Medicago sativa) with increased sucrose phosphate synthase activity shows enhanced growth when grown under N2-fixing conditions.Planta. 2015 Oct;242(4):1009-24. doi: 10.1007/s00425-015-2342-0. Epub 2015 Jun 9. Planta. 2015. PMID: 26055333
-
Carbon and nitrogen metabolism in ectomycorrhizal fungi and ectomycorrhizas.Biochimie. 1987 Jun-Jul;69(6-7):569-81. doi: 10.1016/0300-9084(87)90176-3. Biochimie. 1987. PMID: 3120792 Review.
-
Actinorhizal symbioses and their N2 fixation.New Phytol. 1997 Jul;136(3):375-405. doi: 10.1046/j.1469-8137.1997.00755.x. New Phytol. 1997. PMID: 33863007 Review.
Cited by
-
Insights into carbon and nitrogen metabolism and antioxidant potential during vegetative phase in quinoa (Chenopodium quinoa Willd.).Protoplasma. 2022 Sep;259(5):1301-1319. doi: 10.1007/s00709-022-01736-3. Epub 2022 Jan 22. Protoplasma. 2022. PMID: 35064825
-
The impact of the rhizobia-legume symbiosis on host root system architecture.J Exp Bot. 2020 Jun 26;71(13):3902-3921. doi: 10.1093/jxb/eraa198. J Exp Bot. 2020. PMID: 32337556 Free PMC article. Review.
-
Genetic Engineering and Genome Editing for Improving Nitrogen Use Efficiency in Plants.Cells. 2021 Nov 25;10(12):3303. doi: 10.3390/cells10123303. Cells. 2021. PMID: 34943810 Free PMC article. Review.
-
Biotechnological strategies for improved photosynthesis in a future of elevated atmospheric CO2.Planta. 2019 Nov 29;251(1):24. doi: 10.1007/s00425-019-03301-4. Planta. 2019. PMID: 31784816 Review.
-
Identification of Genetic Loci Associated With Crude Protein Content and Fiber Composition in Alfalfa (Medicago sativa L.) Using QTL Mapping.Front Plant Sci. 2021 Feb 18;12:608940. doi: 10.3389/fpls.2021.608940. eCollection 2021. Front Plant Sci. 2021. PMID: 33679827 Free PMC article.
References
-
- Aleman, L. , Ortega, J. L. , Martinez‐Grimes, M. , Seger, M. , Holguin, F. O. , Uribe, D. J. , & Sengupta‐Gopalan, C. (2010). Nodule‐enhanced expression of a sucrose phosphate synthase gene member (MsSPSA) has a role in carbon and nitrogen metabolism in the nodules of alfalfa (Medicago sativa L.). Planta, 231, 233–244. 10.1007/s00425-009-1043-y - DOI - PMC - PubMed
-
- Amiour, N. , Imbaud, S. , Clément, G. , Agier, N. , Zivy, M. , Valot, B. , & Tercet‐Laforgue, T. (2012). The use of metabolomics integrated with transcriptomic and proteomic studies for identifying key steps involved in the control of nitrogen metabolism in crops such as maize. Journal of Experimental Botany, 63, 5017–5033. 10.1093/jxb/ers186 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

