Chlorophyll catabolism precedes changes in chloroplast structure and proteome during leaf senescence
- PMID: 31245770
- PMCID: PMC6508775
- DOI: 10.1002/pld3.127
Chlorophyll catabolism precedes changes in chloroplast structure and proteome during leaf senescence
Abstract
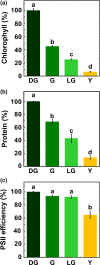
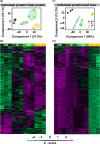
The earliest visual changes of leaf senescence occur in the chloroplast as chlorophyll is degraded and photosynthesis declines. Yet, a comprehensive understanding of the sequence of catabolic events occurring in chloroplasts during natural leaf senescence is still missing. Here, we combined confocal and electron microscopy together with proteomics and biochemistry to follow structural and molecular changes during Arabidopsis leaf senescence. We observed that initiation of chlorophyll catabolism precedes other breakdown processes. Chloroplast size, stacking of thylakoids, and efficiency of PSII remain stable until late stages of senescence, whereas the number and size of plastoglobules increase. Unlike catabolic enzymes, whose level increase, the level of most proteins decreases during senescence, and chloroplast proteins are overrepresented among these. However, the rate of their disappearance is variable, mostly uncoordinated and independent of their inherent stability during earlier developmental stages. Unexpectedly, degradation of chlorophyll-binding proteins lags behind chlorophyll catabolism. Autophagy and vacuole proteins are retained at relatively high levels, highlighting the role of extra-plastidic degradation processes especially in late stages of senescence. The observation that chlorophyll catabolism precedes all other catabolic events may suggest that this process enables or signals further catabolic processes in chloroplasts.
Keywords: Arabidopsis; chlorophyll; chloroplast; photosynthesis; plastoglobule; senescence; thylakoid.
Conflict of interest statement
The authors declare no conflict of interest associated with the work described in this manuscript.
Figures








References
-
- Buchanan‐Wollaston, V. , Page, T. , Harrison, E. , Breeze, E. , Lim, P. O. , Nam, H. G. , … Leaver, C. J. (2005). Comparative transcriptome analysis reveals significant differences in gene expression and signalling pathways between developmental and dark/starvation‐induced senescence in Arabidopsis. Plant Journal, 42, 567–585. 10.1111/j.1365-313X.2005.02399.x - DOI - PubMed
-
- Carrion, C. A. , Costa, M. L. , Martinez, D. E. , Mohr, C. , Humbeck, K. , & Guiamet, J. J. (2013). In vivo inhibition of cysteine proteases provides evidence for the involvement of ‘senescence‐associated vacuoles’ in chloroplast protein degradation during dark‐induced senescence of tobacco leaves. Journal of Experimental Botany, 64, 4967–4980. 10.1093/jxb/ert285 - DOI - PubMed
LinkOut - more resources
Full Text Sources

