Metabolic signatures of cancer cells and stem cells
- PMID: 31245788
- PMCID: PMC6594714
- DOI: 10.1038/s42255-019-0032-0
Metabolic signatures of cancer cells and stem cells
Abstract
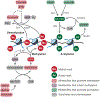
In contrast to terminally differentiated cells, cancer cells and stem cells retain the ability to re-enter the cell cycle and proliferate. In order to proliferate, cells must increase the uptake and catabolism of nutrients to support anabolic cell growth. Intermediates of central metabolic pathways have emerged as key players that can influence cell differentiation 'decisions', processes relevant for both oncogenesis and normal development. Consequently, how cells rewire metabolic pathways to support proliferation may have profound consequences for cellular identity. Here, we discuss the metabolic programs that support proliferation and explore how metabolic states are intimately entwined with the cell fate decisions that characterize stem cells and cancer cells. By comparing the metabolism of pluripotent stem cells and cancer cells, we hope to illuminate common metabolic strategies as well as distinct metabolic features that may represent specialized adaptations to unique cellular demands.
Conflict of interest statement
Competing Interests. A.I. has previously consulted for Foundation Medicine, Inc.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

