Monolayer Sensitivity Enables a 2D IR Spectroscopic Immuno-biosensor for Studying Protein Structures: Application to Amyloid Polymorphs
- PMID: 31246039
- PMCID: PMC6823637
- DOI: 10.1021/acs.jpclett.9b01267
Monolayer Sensitivity Enables a 2D IR Spectroscopic Immuno-biosensor for Studying Protein Structures: Application to Amyloid Polymorphs
Abstract
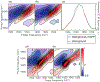
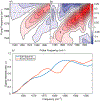
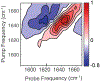
Immunosensors use antibodies to detect and quantify biomarkers of disease, though the sensors often lack structural information. We create a surface-sensitive two-dimensional infrared (2D IR) spectroscopic immunosensor for studying protein structures. We tether antibodies to a plasmonic surface, flow over a solution of amyloid proteins, and measure the 2D IR spectra. The 2D IR spectra provide a global assessment of antigen structure, and isotopically labeled proteins give residue-specific structural information. We report the 2D IR spectra of fibrils and monomers using a polyclonal antibody that targets human islet amyloid polypeptide (hIAPP). We observe two fibrillar polymorphs differing in their structure at the G24 residue, which supports the hypothesis that hIAPP polymorphs form from a common oligomeric intermediate. This work provides insight into the structure of hIAPP, establishes a new method for studying protein structures using 2D IR spectroscopy, and creates a spectroscopic immunoassay applicable for studying a wide range of biomarkers.
Figures

Similar articles
-
Strategies for extracting structural information from 2D IR spectroscopy of amyloid: application to islet amyloid polypeptide.J Phys Chem B. 2009 Nov 26;113(47):15679-91. doi: 10.1021/jp9072203. J Phys Chem B. 2009. PMID: 19883093 Free PMC article.
-
Structural Polymorphs Suggest Competing Pathways for the Formation of Amyloid Fibrils That Diverge from a Common Intermediate Species.Biochemistry. 2018 Nov 20;57(46):6470-6478. doi: 10.1021/acs.biochem.8b00997. Epub 2018 Nov 6. Biochemistry. 2018. PMID: 30375231 Free PMC article.
-
Simultaneously Measured Kinetics of Two Amyloid Polymorphs Using Cross Peak Specific 2D IR Spectroscopy.J Phys Chem Lett. 2023 Dec 28;14(51):11750-11757. doi: 10.1021/acs.jpclett.3c02698. Epub 2023 Dec 20. J Phys Chem Lett. 2023. PMID: 38117179 Free PMC article.
-
Characterisation of the Structure and Oligomerisation of Islet Amyloid Polypeptides (IAPP): A Review of Molecular Dynamics Simulation Studies.Molecules. 2018 Aug 25;23(9):2142. doi: 10.3390/molecules23092142. Molecules. 2018. PMID: 30149632 Free PMC article. Review.
-
Insights into the Conformational Ensemble of Human Islet Amyloid Polypeptide from Molecular Simulations.Curr Pharm Des. 2016;22(23):3601-7. doi: 10.2174/1381612822666160414142538. Curr Pharm Des. 2016. PMID: 27075579 Review.
Cited by
-
Development and Characterization of Electrodes for Surface-Specific Attenuated Total Reflection Two-Dimensional Infrared Spectroelectrochemistry.J Phys Chem C Nanomater Interfaces. 2023 Nov 28;127(48):23199-23211. doi: 10.1021/acs.jpcc.3c05445. eCollection 2023 Dec 7. J Phys Chem C Nanomater Interfaces. 2023. PMID: 38090141 Free PMC article.
-
Structure Changes of a Membrane Polypeptide under an Applied Voltage Observed with Surface-Enhanced 2D IR Spectroscopy.J Phys Chem Lett. 2021 Feb 25;12(7):1786-1792. doi: 10.1021/acs.jpclett.0c03706. Epub 2021 Feb 12. J Phys Chem Lett. 2021. PMID: 33576633 Free PMC article.
-
Coupling chemical biology and vibrational spectroscopy for studies of amyloids in vitro and in cells.Curr Opin Chem Biol. 2021 Oct;64:90-97. doi: 10.1016/j.cbpa.2021.05.005. Epub 2021 Jun 26. Curr Opin Chem Biol. 2021. PMID: 34186291 Free PMC article. Review.
-
Real-Time Monitoring of Chemisorption of Antibodies onto Self-Assembled Monolayers Deposited on Gold Electrodes Using Electrochemical Impedance Spectroscopy.Langmuir. 2025 Jul 1;41(25):15974-15986. doi: 10.1021/acs.langmuir.5c01062. Epub 2025 Jun 17. Langmuir. 2025. PMID: 40524552 Free PMC article.
-
Protein Dynamics by Two-Dimensional Infrared Spectroscopy.Annu Rev Anal Chem (Palo Alto Calif). 2021 Jul 27;14(1):299-321. doi: 10.1146/annurev-anchem-091520-091009. Annu Rev Anal Chem (Palo Alto Calif). 2021. PMID: 34314221 Free PMC article.
References
-
- Cui X; Liu M; Li B Homogeneous Fluorescence-Based Immunoassay via Inner Filter Effect of Gold Nanoparticles on Fluorescence of CdTe Quantum Dots. Analyst 2012, 137, 3293–3299. - PubMed
-
- Kayed R; Leapman RD; Guo Z; Yau W-M; Mattson MP; Tycko R Common Structure of Soluble Amyloid Oligomers Implies Common Mechanism of Pathogenesis. Science 2003, 300, 486–489. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

