Monolayer Sensitivity Enables a 2D IR Spectroscopic Immuno-biosensor for Studying Protein Structures: Application to Amyloid Polymorphs
- PMID: 31246039
- PMCID: PMC6823637
- DOI: 10.1021/acs.jpclett.9b01267
Monolayer Sensitivity Enables a 2D IR Spectroscopic Immuno-biosensor for Studying Protein Structures: Application to Amyloid Polymorphs
Abstract
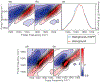
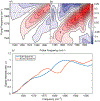
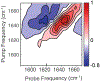
Immunosensors use antibodies to detect and quantify biomarkers of disease, though the sensors often lack structural information. We create a surface-sensitive two-dimensional infrared (2D IR) spectroscopic immunosensor for studying protein structures. We tether antibodies to a plasmonic surface, flow over a solution of amyloid proteins, and measure the 2D IR spectra. The 2D IR spectra provide a global assessment of antigen structure, and isotopically labeled proteins give residue-specific structural information. We report the 2D IR spectra of fibrils and monomers using a polyclonal antibody that targets human islet amyloid polypeptide (hIAPP). We observe two fibrillar polymorphs differing in their structure at the G24 residue, which supports the hypothesis that hIAPP polymorphs form from a common oligomeric intermediate. This work provides insight into the structure of hIAPP, establishes a new method for studying protein structures using 2D IR spectroscopy, and creates a spectroscopic immunoassay applicable for studying a wide range of biomarkers.
Figures

References
-
- Cui X; Liu M; Li B Homogeneous Fluorescence-Based Immunoassay via Inner Filter Effect of Gold Nanoparticles on Fluorescence of CdTe Quantum Dots. Analyst 2012, 137, 3293–3299. - PubMed
-
- Kayed R; Leapman RD; Guo Z; Yau W-M; Mattson MP; Tycko R Common Structure of Soluble Amyloid Oligomers Implies Common Mechanism of Pathogenesis. Science 2003, 300, 486–489. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

