Organ-on-chips made of blood: endothelial progenitor cells from blood reconstitute vascular thromboinflammation in vessel-chips
- PMID: 31246211
- PMCID: PMC6650325
- DOI: 10.1039/c9lc00469f
Organ-on-chips made of blood: endothelial progenitor cells from blood reconstitute vascular thromboinflammation in vessel-chips
Abstract
Development of therapeutic approaches to treat vascular dysfunction and thrombosis at disease- and patient-specific levels is an exciting proposed direction in biomedical research. However, this cannot be achieved with animal preclinical models alone, and new in vitro techniques, like human organ-on-chips, currently lack inclusion of easily obtainable and phenotypically-similar human cell sources. Therefore, there is an unmet need to identify sources of patient primary cells and apply them in organ-on-chips to increase personalized mechanistic understanding of diseases and to assess drugs. In this study, we provide a proof-of-feasibility of utilizing blood outgrowth endothelial cells (BOECs) as a disease-specific primary cell source to analyze vascular inflammation and thrombosis in vascular organ-chips or "vessel-chips". These blood-derived BOECs express several factors that confirm their endothelial identity. The vessel-chips are cultured with BOECs from healthy or diabetic patients and form an intact 3D endothelial lumen. Inflammation of the BOEC endothelium with exogenous cytokines reveals vascular dysfunction and thrombosis in vitro similar to in vivo observations. Interestingly, our study with vessel-chips also reveals that unstimulated BOECs of type 1 diabetic pigs show phenotypic behavior of the disease - high vascular dysfunction and thrombogenicity - when compared to control BOECs or normal primary endothelial cells. These results demonstrate the potential of organ-on-chips made from autologous endothelial cells obtained from blood in modeling vascular pathologies and therapeutic outcomes at a disease and patient-specific level.
Conflict of interest statement
Conflicts of interest
There are no conflicts to declare.
Figures
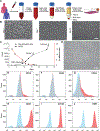



Similar articles
-
Vascular Transcriptomics: Investigating Endothelial Activation and Vascular Dysfunction Using Blood Outgrowth Endothelial Cells, Organ-Chips, and RNA Sequencing.Curr Protoc. 2022 Oct;2(10):e582. doi: 10.1002/cpz1.582. Curr Protoc. 2022. PMID: 36300922 Free PMC article.
-
Neovascularization Potential of Blood Outgrowth Endothelial Cells From Patients With Stable Ischemic Heart Failure Is Preserved.J Am Heart Assoc. 2016 Apr 18;5(4):e002288. doi: 10.1161/JAHA.115.002288. J Am Heart Assoc. 2016. PMID: 27091182 Free PMC article.
-
Embedded macrophages induce intravascular coagulation in 3D blood vessel-on-chip.Biomed Microdevices. 2023 Dec 12;26(1):2. doi: 10.1007/s10544-023-00684-w. Biomed Microdevices. 2023. PMID: 38085384 Free PMC article.
-
Origins and functional differences of blood endothelial cells.Semin Cell Dev Biol. 2024 Mar 1;155(Pt C):23-29. doi: 10.1016/j.semcdb.2023.05.001. Epub 2023 May 16. Semin Cell Dev Biol. 2024. PMID: 37202277 Review.
-
Organs-on-chips technologies - A guide from disease models to opportunities for drug development.Biosens Bioelectron. 2023 Jul 1;231:115271. doi: 10.1016/j.bios.2023.115271. Epub 2023 Mar 31. Biosens Bioelectron. 2023. PMID: 37060819 Review.
Cited by
-
A Potential Role for MAGI-1 in the Bi-Directional Relationship Between Major Depressive Disorder and Cardiovascular Disease.Curr Atheroscler Rep. 2024 Sep;26(9):463-483. doi: 10.1007/s11883-024-01223-5. Epub 2024 Jul 3. Curr Atheroscler Rep. 2024. PMID: 38958925 Free PMC article. Review.
-
Microfluidic endothelium-on-a-chip development, from in vivo to in vitro experimental models.Rom J Morphol Embryol. 2020;61(1):15-23. doi: 10.47162/RJME.61.1.02. Rom J Morphol Embryol. 2020. PMID: 32747891 Free PMC article.
-
Directed self-assembly of a xenogeneic vascularized endocrine pancreas for type 1 diabetes.Nat Commun. 2023 Feb 16;14(1):878. doi: 10.1038/s41467-023-36582-1. Nat Commun. 2023. PMID: 36797282 Free PMC article.
-
Image-based crosstalk analysis of cell-cell interactions during sprouting angiogenesis using blood-vessel-on-a-chip.Stem Cell Res Ther. 2022 Dec 27;13(1):532. doi: 10.1186/s13287-022-03223-1. Stem Cell Res Ther. 2022. PMID: 36575469 Free PMC article.
-
NanoIEA: A Nanopatterned Interdigitated Electrode Array-Based Impedance Assay for Real-Time Measurement of Aligned Endothelial Cell Barrier Functions.Adv Healthc Mater. 2024 Jan;13(2):e2301124. doi: 10.1002/adhm.202301124. Epub 2023 Nov 2. Adv Healthc Mater. 2024. PMID: 37820720 Free PMC article.
References
-
- Creager MA, Circulation, 2016, 133, 2593–2598. - PubMed
-
- Dekkers JF, Wiegerinck CL, de Jonge HR, Bronsveld I, Janssens HM, de Winter-de Groot KM, Brandsma AM, de Jong NWM, Bijvelds MJC, Scholte BJ, Nieuwenhuis EES, van den Brink S, Clevers H, van der Ent CK, Middendorp S and Beekman JM, Nature Medicine, 2013, 19, 939-+. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

