Effect of semaglutide on liver enzymes and markers of inflammation in subjects with type 2 diabetes and/or obesity
- PMID: 31246368
- PMCID: PMC6617813
- DOI: 10.1111/apt.15316
Effect of semaglutide on liver enzymes and markers of inflammation in subjects with type 2 diabetes and/or obesity
Abstract
Background: Obesity and type 2 diabetes are drivers of non-alcoholic fatty liver disease (NAFLD). Glucagon-like peptide-1 analogues effectively treat obesity and type 2 diabetes and may offer potential for NAFLD treatment.
Aim: To evaluate the effect of the glucagon-like peptide-1 analogue, semaglutide, on alanine aminotransferase (ALT) and high-sensitivity C-reactive protein (hsCRP) in subjects at risk of NAFLD.
Methods: Data from a 104-week cardiovascular outcomes trial in type 2 diabetes (semaglutide 0.5 or 1.0 mg/week) and a 52-week weight management trial (semaglutide 0.05-0.4 mg/day) were analysed. Treatment ratios vs placebo were estimated for ALT (both trials) and hsCRP (weight management trial only) using a mixed model for repeated measurements, with or without adjustment for change in body weight.
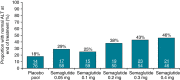
Results: Elevated baseline ALT (men >30 IU/L; women >19 IU/L) was present in 52% (499/957) of weight management trial subjects. In this group with elevated ALT, end-of-treatment ALT reductions were 6%-21% (P<0.05 for doses≥0.2 mg/day) and hsCRP reductions 25%-43% vs placebo (P < 0.05 for 0.2 and 0.4 mg/day). Normalisation of elevated baseline ALT occurred in 25%-46% of weight management trial subjects, vs 18% on placebo. Elevated baseline ALT was present in 41% (1325/3268) of cardiovascular outcomes trial subjects. In this group with elevated ALT, no significant ALT reduction was noted at end-of-treatment for 0.5 mg/week, while a 9% reduction vs placebo was seen for 1.0 mg/week (P = 0.0024). Treatment ratios for changes in ALT and hsCRP were not statistically significant after adjustment for weight change.
Conclusions: Semaglutide significantly reduced ALT and hsCRP in clinical trials in subjects with obesity and/or type 2 diabetes.
© 2019 The Authors. Alimentary Pharmacology & Therapeutics published by John Wiley & Sons Ltd.
Figures





References
-
- Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10:686‐690. - PubMed
-
- Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease‐Meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73‐84. - PubMed
-
- Marchesini G, Brizi M, Bianchi G, et al. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50:1844‐1850. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
