Biodegradable Biliverdin Nanoparticles for Efficient Photoacoustic Imaging
- PMID: 31246412
- PMCID: PMC6903795
- DOI: 10.1021/acsnano.9b01201
Biodegradable Biliverdin Nanoparticles for Efficient Photoacoustic Imaging
Abstract
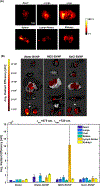
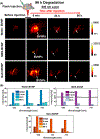
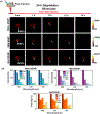
Photoacoustic imaging has emerged as a promising imaging platform with a high tissue penetration depth. However, biodegradable nanoparticles, especially those for photoacoustic imaging, are rare and limited to a few polymeric agents. The development of such nanoparticles holds great promise for clinically translatable diagnostic imaging with high biocompatibility. Metabolically digestible and inherently photoacoustic imaging probes can be developed from nanoprecipitation of biliverdin, a naturally occurring heme-based pigment. The synthesis of nanoparticles composed of a biliverdin network, cross-linked with a bifunctional amine linker, is achieved where spectral tuning relies on the choice of reaction media. Nanoparticles synthesized in water or water containing sodium chloride exhibit higher absorbance and lower fluorescence compared to nanoparticles synthesized in 2-(N-morpholino)ethanesulfonic acid buffer. All nanoparticles display high absorbance at 365 and 680 nm. Excitation at near-infrared wavelengths leads to a strong photoacoustic signal, while excitation with ultraviolet wavelengths results in fluorescence emission. In vivo photoacoustic imaging experiments in mice demonstrated that the nanoparticles accumulate in lymph nodes, highlighting their potential utility as photoacoustic agents for sentinel lymph node detection. The biotransformation of these agents was studied using mass spectroscopy, and they were found to be completely biodegraded in the presence of biliverdin reductase, a ubiquitous enzyme found in the body. Degradation of these particles was also confirmed in vivo. Thus, the nanoparticles developed here are a promising platform for biocompatible biological imaging due to their inherent photoacoustic and fluorescent properties as well as their complete metabolic digestion.
Keywords: biliverdin; biodegradation; bioimaging; fluorescent; nanoparticle; nanoprecipitation; photoacoustic.
Figures







References
-
- Zhang Y; Wang L; Liu L; Lin L; Liu F; Xie Z; Tian H; Chen X. Engineering Metal Organic Frameworks for Photoacoustic Imaging Guided Chemo/Photothermal Combinational Tumor Therapy. ACS Appl Mater. Interfaces 2018, 10, 41035–41045. - PubMed
-
- Qiao Y; Gumin J; Maclellan CJ; Gao F; Bouchard R; Lang FF; Stafford RJ; Melancon MP Magnetic Resonance and Photoacoustic Imaging of Brain Tumor Mediated by Mesenchymal Stem Cell Labeled with Multifunctional Nanoparticle Introduced via Carotid Artery Injection. Nanotechnology 2018, 29, 165101. - PMC - PubMed
-
- Kwon HJ; Shin K; Soh M; Chang H; Kim J; Lee J; Ko G; Kim BH; Kim D; Hyeon T. Large-Scale Synthesis and Medical Applications of Uniform-Sized Metal Oxide Nanoparticles. Adv. Mater. 2018, 30, 1870319. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

