Persistence of the immune response after 4CMenB vaccination, and the response to an additional booster dose in infants, children, adolescents, and young adults
- PMID: 31246520
- PMCID: PMC6930112
- DOI: 10.1080/21645515.2019.1627159
Persistence of the immune response after 4CMenB vaccination, and the response to an additional booster dose in infants, children, adolescents, and young adults
Abstract
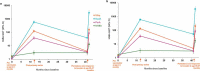
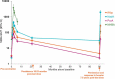
The multicomponent meningococcal serogroup B vaccine, 4CMenB, has demonstrated effectiveness in preventing invasive MenB disease in infants and in controlling MenB outbreaks. The need for/timing of additional booster doses is not yet established. We reviewed eight studies that evaluated antibody persistence and booster following primary 4CMenB vaccination of infants, children, adolescents, and young adults. Putative seroprotective hSBA titers for ≥1 vaccine antigen were maintained by 76-100% of children 24-36 months after priming during infancy and in 84-100% after priming in the second year of life. hSBA levels were higher in vaccinees at 4 and 7.5 years following priming during adolescence than in vaccine-naïve individuals of a similar age. Antibodies persisted at higher levels to NHBA and NadA than to PorA or fHbp. Booster vaccination induced robust anamnestic responses, demonstrating effective priming by 4CMenB across age-groups. These data can inform decision-making to optimize vaccination strategies.
Keywords: 4CMenB; Neisseria meningitidis; antibody persistence; immunogenicity; meningococcal serogroup B.
Figures

References
-
- ECDC European centre for disease prevention and control. Surveillance of invasive bacterial diseases in Europe; 2012. [accessed 2017 October20] http://www.ecdc.europa.eu/en/publications/_layouts/forms/Publication_Dis....
-
- Borrow R, Alarcon P, Carlos J, Caugant DA, Christensen H, Debbag R, De Wals P, Echaniz-Aviles G, Findlow J, Head C, et al. The global meningococcal initiative: global epidemiology, the impact of vaccines on meningococcal disease and the importance of herd protection. Expert Rev Vaccines. 2017;16:313–28. doi: 10.1080/14760584.2017.1258308. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
