Fasting prevents hypoxia-induced defects of proteostasis in C. elegans
- PMID: 31246952
- PMCID: PMC6619831
- DOI: 10.1371/journal.pgen.1008242
Fasting prevents hypoxia-induced defects of proteostasis in C. elegans
Abstract
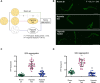
Low oxygen conditions (hypoxia) can impair essential physiological processes and cause cellular damage and death. We have shown that specific hypoxic conditions disrupt protein homeostasis in C. elegans, leading to protein aggregation and proteotoxicity. Here, we show that nutritional cues regulate this effect of hypoxia on proteostasis. Animals fasted prior to hypoxic exposure develop dramatically fewer polyglutamine protein aggregates compared to their fed counterparts, indicating that the effect of hypoxia is abrogated. Fasting also reduced the hypoxia-induced exaggeration of proteostasis defects in animals that express Aβ1-42 and in animals with a temperature-sensitive mutation in dyn-1, suggesting that this effect was not specific to polyglutamine proteins. Our data also demonstrate that the nutritional environment experienced at the onset of hypoxia dictates at least some aspects of the physiological response to hypoxia. We further demonstrate that the insulin/IGF-like signaling pathway plays a role in mediating the protective effects of fasting in hypoxia. Animals with mutations in daf-2, the C. elegans insulin-like receptor, display wild-type levels of hypoxia-induced protein aggregation upon exposure to hypoxia when fed, but are not protected by fasting. DAF-2 acts independently of the FOXO transcription factor, DAF-16, to mediate the protective effects of fasting. These results suggest a non-canonical role for the insulin/IGF-like signaling pathway in coordinating the effects of hypoxia and nutritional state on proteostasis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Contestabile A (2009) Benefits of caloric restriction on brain aging and related pathological States: understanding mechanisms to devise novel therapies. Curr Med Chem 16: 350–361. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

