Mitochondria as central hub of the immune system
- PMID: 31247505
- PMCID: PMC6598836
- DOI: 10.1016/j.redox.2019.101255
Mitochondria as central hub of the immune system
Abstract
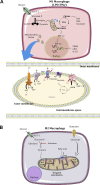
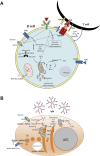
Nearly 130 years after the first insights into the existence of mitochondria, new rolesassociated with these organelles continue to emerge. As essential hubs that dictate cell fate, mitochondria integrate cell physiology, signaling pathways and metabolism. Thus, recent research has focused on understanding how these multifaceted functions can be used to improve inflammatory responses and prevent cellular dysfunction. Here, we describe the role of mitochondria on the development and function of immune cells, highlighting metabolic aspects and pointing out some metabolic- independent features of mitochondria that sustain cell function.
Keywords: And immune cells; Cell fate; Immunometabolism; Mitochondrial function.
Copyright © 2019 The Authors. Published by Elsevier B.V. All rights reserved.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

