Tubular NOX4 expression decreases in chronic kidney disease but does not modify fibrosis evolution
- PMID: 31247506
- PMCID: PMC6598841
- DOI: 10.1016/j.redox.2019.101234
Tubular NOX4 expression decreases in chronic kidney disease but does not modify fibrosis evolution
Abstract
Background: NADPH oxidase 4 (NOX4) catalyzes the formation of hydrogen peroxide (H2O2). NOX4 is highly expressed in the kidney, but its role in renal injury is unclear and may depend on its specific tissue localization.
Methods: We performed immunostaining with a specific anti-NOX4 antibody and measured NOX4 mRNA expression in human renal biopsies encompassing diverse renal diseases. We generated transgenic mice specifically overexpressing mouse Nox4 in renal tubular cells and subjected the animals to the unilateral ureteral obstruction (UUO) model of fibrosis.
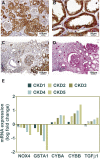
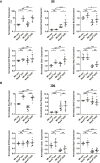
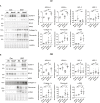
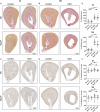
Results: In normal human kidney, NOX4 protein expression was at its highest on the basolateral side of proximal tubular cells. NOX4 expression increased in mesangial cells and podocytes in proliferative diabetic nephropathy. In tubular cells, NOX4 protein expression decreased in all types of chronic renal disease studied. This finding was substantiated by decreased NOX4 mRNA expression in the tubulo-interstitial compartment in a repository of 175 human renal biopsies. Overexpression of tubular NOX4 in mice resulted in enhanced renal production of H2O2, increased NRF2 protein expression and decreased glomerular filtration, likely via stimulation of the tubulo-glomerular feedback. Tubular NOX4 overexpression had no obvious impact on kidney morphology, apoptosis, or fibrosis at baseline. Under acute and chronic tubular injury induced by UUO, overexpression of NOX4 in tubular cells did not modify the course of the disease.
Conclusions: NOX4 expression was decreased in tubular cells in all types of CKD tested. Tubular NOX4 overexpression did not induce injury in the kidney, and neither modified microvascularization, nor kidney structural lesions in fibrosis.
Keywords: Chronic kidney disease; Diabetes; IgA nephropathy; Kidney fibrosis; NOX4; Renal biopsy; Tubular cells.
Copyright © 2019 The Authors. Published by Elsevier B.V. All rights reserved.
Figures




References
-
- Diebold L., Chandel N.S. Mitochondrial ROS regulation of proliferating cells. Free Radic. Biol. Med. 2016;100:86–93. - PubMed
-
- Garcia-Redondo A.B., Aguado A., Briones A.M., Salaices M. NADPH oxidases and vascular remodeling in cardiovascular diseases. Pharmacol. Res. 2016;114:110–120. - PubMed
-
- Rajaram R.D., Dissard R., Jaquet V., de Seigneux S. Potential benefits and harms of NADPH oxidase type 4 in the kidneys and cardiovascular system. Nephrol. Dial. Transplant. 2018;34(4):567–576. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

