Re2O7-Catalyzed Approach to Spirocyclic Ether Formation from Acyclic Precursors: Observation of Remote Stereoinduction
- PMID: 31247770
- PMCID: PMC7720882
- DOI: 10.1021/acs.orglett.9b01660
Re2O7-Catalyzed Approach to Spirocyclic Ether Formation from Acyclic Precursors: Observation of Remote Stereoinduction
Abstract
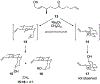
Ketones that are flanked by an allylic alcohol and an alkene isomerize to spirocyclic ethers in the presence of Re2O7 through allylic alcohol transposition, oxocarbenium ion formation, and Prins cyclization. These processes provide significant increases in molecular complexity, with multiple stereocenters being set relative to a stereocenter in the substrate. Stereoselectivity arises from the initial reversible steps being more rapid than the final step, thereby allowing for thermodynamically controlled stereochemical equilibration prior to product formation.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





Similar articles
-
Regiochemical control in the metal-catalyzed transposition of allylic silyl ethers.J Am Chem Soc. 2006 Jun 28;128(25):8142-3. doi: 10.1021/ja0620639. J Am Chem Soc. 2006. PMID: 16787071 Free PMC article.
-
Synthesis of Spirocyclic Ethers by Enantioselective Copper-Catalyzed Carboetherification of Alkenols.Angew Chem Int Ed Engl. 2018 Sep 24;57(39):12921-12924. doi: 10.1002/anie.201808554. Epub 2018 Sep 3. Angew Chem Int Ed Engl. 2018. PMID: 30117646 Free PMC article.
-
Dehydrative Re2O7-Catalyzed Approach to Dihydropyran Synthesis.Org Lett. 2020 Dec 18;22(24):9513-9517. doi: 10.1021/acs.orglett.0c03526. Epub 2020 Dec 9. Org Lett. 2020. PMID: 33295777
-
Rapid and stereoselective synthesis of spirocyclic ethers via the intramolecular Piancatelli rearrangement.Org Lett. 2013 Feb 1;15(3):476-9. doi: 10.1021/ol303263q. Epub 2013 Jan 23. Org Lett. 2013. PMID: 23343463
-
A Review of Approaches Developed for Spiroether Synthesis.Top Curr Chem (Cham). 2025 Jun 9;383(3):24. doi: 10.1007/s41061-025-00508-w. Top Curr Chem (Cham). 2025. PMID: 40489017 Review.
Cited by
-
Recent synthetic strategies toward the synthesis of spirocyclic compounds comprising six-membered carbocyclic/heterocyclic ring systems.Mol Divers. 2021 Nov;25(4):2487-2532. doi: 10.1007/s11030-020-10126-x. Epub 2020 Jul 21. Mol Divers. 2021. PMID: 32696299 Review.
-
Recent Advances in the Prins Reaction.ACS Omega. 2022 Aug 31;7(36):31621-31627. doi: 10.1021/acsomega.2c04765. eCollection 2022 Sep 13. ACS Omega. 2022. PMID: 36120064 Free PMC article. Review.
-
Dictating Thermodynamic Control through Tethering: Applications to Stereoselective Bis-Spiroketal Synthesis.Angew Chem Int Ed Engl. 2020 Apr 16;59(16):6622-6626. doi: 10.1002/anie.201916270. Epub 2020 Feb 25. Angew Chem Int Ed Engl. 2020. PMID: 31991022 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

