Growth, Feeding Tolerance and Metabolism in Extreme Preterm Infants under an Exclusive Human Milk Diet
- PMID: 31248006
- PMCID: PMC6683272
- DOI: 10.3390/nu11071443
Growth, Feeding Tolerance and Metabolism in Extreme Preterm Infants under an Exclusive Human Milk Diet
Abstract
Background: For preterm infants, human milk (HM) has to be fortified to cover their enhanced nutritional requirements and establish adequate growth. Most HM fortifiers are based on bovine protein sources (BMF). An HM fortifier based on human protein sources (HMF) has become available in the last few years. The aim of this study is to investigate the impact of an HMF versus BMF on growth in extremely low birth weight (ELBW, <1000 g) infants.
Methods: This was a retrospective, controlled, multicenter cohort study in infants with a birthweight below 1000 g. The HMF group received an exclusive HM diet up to 32+0 weeks of gestation and was changed to BMF afterwards. The BMF group received HM+BMF from fortifier introduction up to 37+0 weeks.
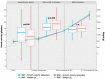
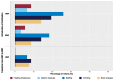
Results: 192 extremely low birth weight (ELBW)-infants were included (HMF n = 96, BMF n = 96) in the study. After the introduction of fortification, growth velocity up to 32+0 weeks was significantly lower in the HMF group (16.5 g/kg/day) in comparison to the BMF group (18.9 g/kg/day, p = 0.009) whereas all other growth parameters did not differ from birth up to 37+0 weeks. Necrotizing enterocolitis (NEC) incidence was 10% in the HMF and 8% in the BMF group.
Conclusion: Results from this study do not support the superiority of HFM over BMF in ELBW infants.
Keywords: ELBW-infants; exclusive human milk diet; feeding tolerance; fortification of human milk; growth velocity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Growth, efficacy, and safety of feeding an iron-fortified human milk fortifier.Pediatrics. 2004 Dec;114(6):e699-706. doi: 10.1542/peds.2004-0911. Epub 2004 Nov 15. Pediatrics. 2004. PMID: 15545616 Clinical Trial.
-
Concentrated Preterm Formula as a Liquid Human Milk Fortifier at Initiation Stage in Extremely Low Birth Weight Preterm Infants: Short Term and 2-year Follow-up Outcomes.Nutrients. 2020 Jul 26;12(8):2229. doi: 10.3390/nu12082229. Nutrients. 2020. PMID: 32722642 Free PMC article.
-
Pilot study on growth parameters and nutritional biochemical markers in very low birth weight preterm infants fed human milk fortified with either human milk fortifier or post discharge formula.J Med Assoc Thai. 2014 Jun;97 Suppl 6:S164-75. J Med Assoc Thai. 2014. PMID: 25391190 Clinical Trial.
-
Association of Fortification with Human Milk versus Bovine Milk-Based Fortifiers on Short-Term Outcomes in Preterm Infants-A Meta-Analysis.Nutrients. 2024 Mar 21;16(6):910. doi: 10.3390/nu16060910. Nutrients. 2024. PMID: 38542821 Free PMC article. Review.
-
Nutritional interventions to reduce rates of infection, necrotizing enterocolitis and mortality in very preterm infants.Pediatr Res. 2020 Jan;87(2):371-377. doi: 10.1038/s41390-019-0630-2. Epub 2019 Oct 23. Pediatr Res. 2020. PMID: 31645057
Cited by
-
Early fortification of enteral feedings for infants <1250 grams birth weight receiving a human milk diet including human milk based fortifier.J Neonatal Perinatal Med. 2020;13(2):215-221. doi: 10.3233/NPM-190300. J Neonatal Perinatal Med. 2020. PMID: 31707377 Free PMC article.
-
Exclusive human milk diet is associated with lower risk of motor function impairment at three years of corrected age.J Perinatol. 2025 Apr 21. doi: 10.1038/s41372-025-02296-z. Online ahead of print. J Perinatol. 2025. PMID: 40259098
-
[Clinical guidelines for the diagnosis and treatment of feeding intolerance in preterm infants (2020)].Zhongguo Dang Dai Er Ke Za Zhi. 2020 Oct;22(10):1047-1055. doi: 10.7499/j.issn.1008-8830.2008132. Zhongguo Dang Dai Er Ke Za Zhi. 2020. PMID: 33059799 Free PMC article. Chinese.
-
Fortification With Bovine Colostrum Enhances Antibacterial Activity of Human Milk.JPEN J Parenter Enteral Nutr. 2021 Sep;45(7):1417-1424. doi: 10.1002/jpen.2060. Epub 2021 Feb 11. JPEN J Parenter Enteral Nutr. 2021. PMID: 33305396 Free PMC article.
-
Investigating the effect of oropharyngeal colostrum in the prevention of late-onset sepsis in preterm infants: a randomized controlled trial.Sci Rep. 2025 May 19;15(1):17390. doi: 10.1038/s41598-025-02309-z. Sci Rep. 2025. PMID: 40389508 Free PMC article. Clinical Trial.
References
-
- Agostoni C., Buonocore G., Carnielli V.P., De Curtis M., Darmaun D., Decsi T., Domellöf M., Embleton N.D., Fusch C., Genzel-Boroviczeny O., et al. Enteral nutrient supply for preterm infants: Commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2010;50:85–91. doi: 10.1097/MPG.0b013e3181adaee0. - DOI - PubMed
-
- Horbar J.D., Ehrenkranz R.A., Badger G.J., Edwards E.M., Morrow K.A., Soll R.F., Buzas J.S., Bertino E., Gagliardi L., Bellù R., et al. Weight Growth Velocity and Postnatal Growth Failure in Infants 501 to 1500 Grams: 2000–2013. Pediatrics. 2015;136:e84–e92. doi: 10.1542/peds.2015-0129. - DOI - PubMed
-
- Nutrition AAoPCo Nutritional needs of Low-Birth-Weight Infants. Pediatrics. 1977;60:519–530. - PubMed
-
- Nutrition AAoPCo Nutritional Needs of Low-Birth-Weight Infants. Pediatrics. 1985;75:976–986. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

