Biocompatible and Antimicrobial Electrospun Membranes Based on Nanocomposites of Chitosan/Poly (Vinyl Alcohol)/Graphene Oxide
- PMID: 31248075
- PMCID: PMC6627348
- DOI: 10.3390/ijms20122987
Biocompatible and Antimicrobial Electrospun Membranes Based on Nanocomposites of Chitosan/Poly (Vinyl Alcohol)/Graphene Oxide
Abstract
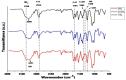
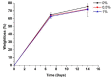
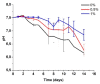
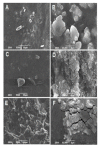
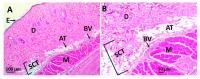
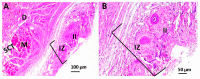
Tissue engineering is gaining attention rapidly to replace and repair defective tissues in the human body after illnesses and accidents in different organs. Electrospun nanofiber scaffolds have emerged as a potential alternative for cell regeneration and organ replacement. In this paper, porous membranes, based on nanofibrous chitosan (CS), polyvinyl alcohol (PVA), and graphene oxide (GO), were obtained via electrospinning methodology. Three different formulations were obtained varying GO content, being characterized by Fourier Transform Infrared spectroscopy (FTIR), scanning electron microscopy (SEM), and energy dispersive spectroscopy (EDS). In vitro tests were carried out, consisting of hydrolytic degradation inside simulated biological fluid (SBF), and in vivo tests were carried out, where the material was implanted in Wistar rats' subcutaneous tissue to determine its biocompatibility. The antibacterial activity was tested against Gram-positive bacteria Bacillus cereus and Staphylococcus aureus, and against Gram-negative Salmonella enterica and Escherichia coli, by contact of the electrospun nanofiber scaffolds above inoculum bacterial in Müeller Hinton agar with good inhibition only for scaffolds with the higher GO content (1.0%). The results confirmed good biocompatibility of the nanofibrous scaffolds after in vivo tests in Wistar rats, which evidences its high potential in applications of tissue regeneration.
Keywords: antibacterial nanofibrous membranes; chitosan; electrospinning; graphene oxide; polyvinyl alcohol.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Antimicrobial Films Based on Nanocomposites of Chitosan/Poly(vinyl alcohol)/Graphene Oxide for Biomedical Applications.Biomolecules. 2019 Mar 18;9(3):109. doi: 10.3390/biom9030109. Biomolecules. 2019. PMID: 30889930 Free PMC article.
-
Enhanced antimicrobial activity and pH-responsive sustained release of chitosan/poly (vinyl alcohol)/graphene oxide nanofibrous membrane loading with allicin.Int J Biol Macromol. 2020 Oct 15;161:1405-1413. doi: 10.1016/j.ijbiomac.2020.08.051. Epub 2020 Aug 10. Int J Biol Macromol. 2020. PMID: 32791265
-
Novel electrospun chitosan/polyvinyl alcohol/zinc oxide nanofibrous mats with antibacterial and antioxidant properties for diabetic wound healing.Int J Biol Macromol. 2018 Dec;120(Pt A):385-393. doi: 10.1016/j.ijbiomac.2018.08.057. Epub 2018 Aug 12. Int J Biol Macromol. 2018. PMID: 30110603
-
Biomedical applications of chitosan electrospun nanofibers as a green polymer - Review.Carbohydr Polym. 2019 Mar 1;207:588-600. doi: 10.1016/j.carbpol.2018.12.011. Epub 2018 Dec 8. Carbohydr Polym. 2019. PMID: 30600043 Review.
-
Electrospun chitosan membranes containing bioactive and therapeutic agents for enhanced wound healing.Int J Biol Macromol. 2020 Aug 1;156:153-170. doi: 10.1016/j.ijbiomac.2020.03.207. Epub 2020 Mar 27. Int J Biol Macromol. 2020. PMID: 32229203 Review.
Cited by
-
Enhancement of 5-Fluorouracil Drug Delivery in a Graphene Oxide Containing Electrospun Chitosan/Polyvinylpyrrolidone Construct.Materials (Basel). 2024 Oct 31;17(21):5300. doi: 10.3390/ma17215300. Materials (Basel). 2024. PMID: 39517573 Free PMC article.
-
Recent advances in nanoantibiotics against multidrug-resistant bacteria.Nanoscale Adv. 2023 Oct 5;5(23):6278-6317. doi: 10.1039/d3na00530e. eCollection 2023 Nov 21. Nanoscale Adv. 2023. PMID: 38024316 Free PMC article. Review.
-
Synthesis of Chitosan Beads Incorporating Graphene Oxide/Titanium Dioxide Nanoparticles for In Vivo Studies.Molecules. 2020 May 14;25(10):2308. doi: 10.3390/molecules25102308. Molecules. 2020. PMID: 32423061 Free PMC article.
-
Recent Progress and Trends in the Development of Electrospun and 3D Printed Polymeric-Based Materials to Overcome Antimicrobial Resistance (AMR).Pharmaceutics. 2023 Jul 16;15(7):1964. doi: 10.3390/pharmaceutics15071964. Pharmaceutics. 2023. PMID: 37514150 Free PMC article. Review.
-
Electrospun PVA Fibers for Drug Delivery: A Review.Polymers (Basel). 2023 Sep 20;15(18):3837. doi: 10.3390/polym15183837. Polymers (Basel). 2023. PMID: 37765691 Free PMC article. Review.
References
-
- Abd-Khorsand S., Saber-Samandari S., Saber-Samandari S. Development of nanocomposite scaffolds based on TiO2 doped in grafted chitosan/hydroxyapatite by freeze-drying method and evaluation of biocompatibility. Int. J. Biol. Macromol. 2017;101:51–58. doi: 10.1016/j.ijbiomac.2017.03.067. - DOI - PubMed
-
- Wu S., Liu X., Yeung K.W.K., Liu C., Yang X. Biomimetic porous scaffolds for bone tissue engineering. Mater. Sci. Eng. R Rep. 2014;80:1–36. doi: 10.1016/j.mser.2014.04.001. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

