Circulating Concentrations of GDF11 are Positively Associated with TSH Levels in Humans
- PMID: 31248139
- PMCID: PMC6617068
- DOI: 10.3390/jcm8060878
Circulating Concentrations of GDF11 are Positively Associated with TSH Levels in Humans
Abstract
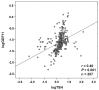
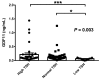
Growth differentiation factor 11 (GDF11) is a member of the transforming growth factor (TGF)-β superfamily which declines with age and has been proposed as an anti-aging factor with regenerative effects in skeletal muscle in mice. However, recent data in humans and mice are conflicting, casting doubts about its true functional actions. The aim of the present study was to analyze the potential involvement of GFD11 in energy homeostasis in particular in relation with thyroid hormones. Serum concentrations of GDF11 were measured by enzyme-linked immunosorbent assay (ELISA) in 287 subjects. A highly significant positive correlation was found between GDF11 and thyroid-stimulating hormone (TSH) concentrations (r = 0.40, p < 0.001). Neither resting energy expenditure (REE) nor REE per unit of fat-free mass (REE/FFM) were significantly correlated (p > 0.05 for both) with GDF11 levels. In a multiple linear regression analysis, the model that best predicted logGDF11 included logTSH, leptin, body mass index (BMI), age, and C-reactive protein (logCRP). This model explained 37% of the total variability of logGDF11 concentrations (p < 0.001), with only logTSH being a significant predictor of logGDF11. After segregating subjects by TSH levels, those within the low TSH group exhibited significantly decreased (p < 0.05) GDF11 concentrations as compared to the normal TSH group or the high TSH group. A significant correlation of GDF11 levels with logCRP (r = 0.19, p = 0.025) was found. GDF11 levels were not related to the presence of hypertension or cardiopathy. In conclusion, our results show that circulating concentrations of GDF11 are closely associated with TSH concentrations and reduced in subjects with low TSH levels. However, GDF11 is not related to the regulation of energy expenditure. Our data also suggest that GDF11 may be involved in the regulation of inflammation, without relation to cardiac function. Further research is needed to elucidate the role of GDF11 in metabolism and its potential involvement in thyroid pathophysiology.
Keywords: CRP; GDF11; REE; TSH; leptin.
Conflict of interest statement
The authors have nothing to disclose. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
Similar articles
-
GDF11 Implications in Cancer Biology and Metabolism. Facts and Controversies.Front Oncol. 2019 Oct 15;9:1039. doi: 10.3389/fonc.2019.01039. eCollection 2019. Front Oncol. 2019. PMID: 31681577 Free PMC article. Review.
-
Circulating GDF11 levels are decreased with age but are unchanged with obesity and type 2 diabetes.Aging (Albany NY). 2019 Mar 21;11(6):1733-1744. doi: 10.18632/aging.101865. Aging (Albany NY). 2019. PMID: 30897065 Free PMC article.
-
Subclinical hypothyroidism in obese patients: relation to resting energy expenditure, serum leptin, body composition, and lipid profile.Obes Res. 2001 Mar;9(3):196-201. doi: 10.1038/oby.2001.21. Obes Res. 2001. PMID: 11323445
-
Association of Thyroid-Stimulating Hormone with Resting Energy Expenditure in Euthyroid Elderly Subjects: A Cross-Sectional Study.Ann Nutr Metab. 2016;68(1):12-8. doi: 10.1159/000441625. Epub 2015 Nov 11. Ann Nutr Metab. 2016. PMID: 26555616
-
The role of GDF11 in aging and skeletal muscle, cardiac and bone homeostasis.Crit Rev Biochem Mol Biol. 2019 Apr;54(2):174-183. doi: 10.1080/10409238.2019.1610722. Crit Rev Biochem Mol Biol. 2019. PMID: 31144559 Review.
Cited by
-
Growth differentiation factor 11: a "rejuvenation factor" involved in regulation of age-related diseases?Aging (Albany NY). 2021 Apr 22;13(8):12258-12272. doi: 10.18632/aging.202881. Epub 2021 Apr 22. Aging (Albany NY). 2021. PMID: 33886503 Free PMC article. Review.
-
Resting Energy Expenditure Is Not Altered in Children and Adolescents with Obesity. Effect of Age and Gender and Association with Serum Leptin Levels.Nutrients. 2021 Apr 7;13(4):1216. doi: 10.3390/nu13041216. Nutrients. 2021. PMID: 33917063 Free PMC article.
-
GDF11 induces mild hepatic fibrosis independent of metabolic health.Aging (Albany NY). 2020 Oct 28;12(20):20024-20046. doi: 10.18632/aging.104182. Epub 2020 Oct 28. Aging (Albany NY). 2020. PMID: 33126224 Free PMC article.
-
GDF11 Implications in Cancer Biology and Metabolism. Facts and Controversies.Front Oncol. 2019 Oct 15;9:1039. doi: 10.3389/fonc.2019.01039. eCollection 2019. Front Oncol. 2019. PMID: 31681577 Free PMC article. Review.
-
Novel insights into the pleiotropic health effects of growth differentiation factor 11 gained from genome-wide association studies in population biobanks.BMC Genomics. 2024 Sep 6;25(1):837. doi: 10.1186/s12864-024-10710-7. BMC Genomics. 2024. PMID: 39237910 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

