Amelioration of Behavioral Impairments and Neuropathology by Antiepileptic Drug Topiramate in a Transgenic Alzheimer's Disease Model Mice, APP/PS1
- PMID: 31248209
- PMCID: PMC6628361
- DOI: 10.3390/ijms20123003
Amelioration of Behavioral Impairments and Neuropathology by Antiepileptic Drug Topiramate in a Transgenic Alzheimer's Disease Model Mice, APP/PS1
Abstract
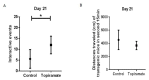
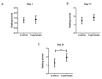
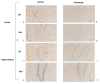
Alzheimer's disease (AD) is a neurodegenerative disease that is the main cause of dementia in the elderly. The aggregation of β-amyloid peptides is one of the characterizing pathological changes of AD. Topiramate is an antiepileptic drug, which in addition, is used in the treatment of many neuropsychiatric disorders. In this study, the therapeutic effects of topiramate were investigated in a transgenic mouse model of cerebral amyloidosis (APP/PS1 mice). Before, during, and after topiramate treatment, behavioral tests were performed. Following a treatment period of 21 days, topiramate significantly ameliorated deficits in nest-constructing capability as well as in social interaction. Thereafter, brain sections of mice were analyzed, and a significant attenuation of microglial activation as well as β-amyloid deposition was observed in sections from topiramate-treated APP/PS1 mice. Therefore, topiramate could be considered as a promising drug in the treatment of human AD.
Keywords: APP/PS1 transgenic mouse; Alzheimer’s disease; amyloidosis; topiramate.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Hesperidin ameliorates behavioral impairments and neuropathology of transgenic APP/PS1 mice.Behav Brain Res. 2015 Mar 15;281:32-42. doi: 10.1016/j.bbr.2014.12.012. Epub 2014 Dec 12. Behav Brain Res. 2015. PMID: 25510196
-
Long-term treadmill exercise inhibits the progression of Alzheimer's disease-like neuropathology in the hippocampus of APP/PS1 transgenic mice.Behav Brain Res. 2013 Nov 1;256:261-72. doi: 10.1016/j.bbr.2013.08.008. Epub 2013 Aug 19. Behav Brain Res. 2013. PMID: 23968591
-
Triptolide Rescues Spatial Memory Deficits and Amyloid-β Aggregation Accompanied by Inhibition of Inflammatory Responses and MAPKs Activity in APP/PS1 Transgenic Mice.Curr Alzheimer Res. 2016;13(3):288-96. doi: 10.2174/156720501303160217122803. Curr Alzheimer Res. 2016. PMID: 26906357
-
Behavioural and psychological symptoms of dementia in mouse models of Alzheimer's disease-related pathology.Neurosci Biobehav Rev. 2020 May;112:634-647. doi: 10.1016/j.neubiorev.2020.02.012. Epub 2020 Feb 15. Neurosci Biobehav Rev. 2020. PMID: 32070692 Review.
-
Transgenic mouse models of Alzheimer disease: developing a better model as a tool for therapeutic interventions.Curr Pharm Des. 2012;18(8):1131-47. doi: 10.2174/138161212799315786. Curr Pharm Des. 2012. PMID: 22288400 Free PMC article. Review.
Cited by
-
Dl-3-n-butylphthalide alleviates cognitive impairment in amyloid precursor protein/presenilin 1 transgenic mice by regulating the striatal-enriched protein tyrosine phosphatase/ERK/cAMP-response element-binding protein signaling pathway.Exp Ther Med. 2022 May;23(5):319. doi: 10.3892/etm.2022.11248. Epub 2022 Mar 10. Exp Ther Med. 2022. PMID: 35350668 Free PMC article.
-
Alzheimer's disease-associated genotypes differentially influence chronic evoked seizure outcomes and antiseizure medicine efficacy in aged mice.J Alzheimers Dis. 2025 Jul;106(2):547-561. doi: 10.1177/13872877251343321. Epub 2025 Jul 1. J Alzheimers Dis. 2025. PMID: 40458037
-
Alzheimer's disease and epilepsy: An increasingly recognized comorbidity.Front Aging Neurosci. 2022 Nov 10;14:940515. doi: 10.3389/fnagi.2022.940515. eCollection 2022. Front Aging Neurosci. 2022. PMID: 36438002 Free PMC article. Review.
-
Zerumbone ameliorates behavioral impairments and neuropathology in transgenic APP/PS1 mice by suppressing MAPK signaling.J Neuroinflammation. 2020 Feb 17;17(1):61. doi: 10.1186/s12974-020-01744-1. J Neuroinflammation. 2020. PMID: 32066466 Free PMC article.
-
Alzheimer's disease-associated genotypes differentially influence chronic evoked seizure outcomes and antiseizure medicine activity in aged mice.bioRxiv [Preprint]. 2024 Oct 7:2024.10.06.616921. doi: 10.1101/2024.10.06.616921. bioRxiv. 2024. Update in: J Alzheimers Dis. 2025 Jul;106(2):547-561. doi: 10.1177/13872877251343321. PMID: 39416203 Free PMC article. Updated. Preprint.
References
-
- Fu H., Li W., Luo J., Lee N.T.K., Li M., Tsim K.W.K., Pang Y., Youdim M.B.H., Han Y. Promising anti-Alzheimer’s dimer bis(7)-tacrine reduces beta-amyloid generation by directly inhibiting BACE-1 activity. Biochem. Biophys. Res. Commun. 2008;366:631–636. doi: 10.1016/j.bbrc.2007.11.068. - DOI - PubMed
-
- Qing H., He G., Ly P.T.T., Fox C.J., Staufenbiel M., Cai F., Zhang Z., Wei S., Sun X., Chen C.-H., et al. Valproic acid inhibits Abeta production, neuritic plaque formation, and behavioral deficits in Alzheimer’s disease mouse models. J. Exp. Med. 2008;205:2781–2789. doi: 10.1084/jem.20081588. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

