Implementing reflective multicriteria decision analysis (MCDA) to assess orphan drugs value in the Catalan Health Service (CatSalut)
- PMID: 31248421
- PMCID: PMC6598260
- DOI: 10.1186/s13023-019-1121-6
Implementing reflective multicriteria decision analysis (MCDA) to assess orphan drugs value in the Catalan Health Service (CatSalut)
Abstract
Background: Orphan medicines show some characteristics that hinder the evaluation of their clinical added value. The often low level of evidence available for orphan drugs, together with a high budget impact and an incremental cost-effectiveness ratio many times higher than drugs used for non-orphan diseases, represent challenges in their appraisal and effective access to clinical use. In order to explore how to handle these hurdles, the Catalan Health Service (CatSalut) began an initiative on a multidimensional assessment of drugs value during the appraisal process. Reflective multicriteria decision analysis (MCDA) using analytical methods was chosen, since it may help to standardise and contextualize all the relevant data related with the drug that could contribute to a decision. The aim of the study was to determine whether the implementation of reflective MCDA methodology could support the decision-making process about orphan medicines in the context of CatSalut.
Methods: The assessment and decision-making process for orphan drugs in the Programa d'Harmonització Farmacoterapeutica (PHF) of CatSalut was prioritized to test the implementation of the reflective MCDA both a qualitative and quantitatively. A staged approach was used with the following main steps: selection and structuration of quantitative criteria (Core Model) and qualitative criteria (Contextual Tool), framework scoring and assessment of three orphan drug case studies. This proof-of-concept would grant a continued refinement of the methodology and, if and when validated, its potential integration to other therapeutic areas of the PHF.
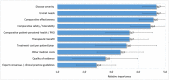
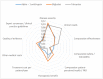
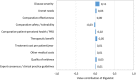
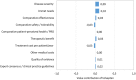
Results: The final framework was composed by 10 quantitative criteria (Core Model) and 4 qualitative criteria (Contextual Tool) according to the PHF goals being the most important criteria "disease severity", "unmet need", "comparative effectiveness" and "comparative safety /tolerability". The matrix developed for the case studies served as a guide for the selection of the essential information that the decision-makers were expected to include in a framework. The reflective discussion was considered the most relevant phase of the approach to support inputs for health decision-making processes reflecting both drug value and place in therapy.
Conclusions: The study showed that reflective MCDA methodology could be implemented to complement the decision-making process in CatSalut, as an aid to determine the clinical added value for orphan medicines. MCDA provided transparency and a structured discussion during the committee meetings, thus increasing transparency and predictability of the relevant items supporting the agreements adopted on orphan drugs access.
Keywords: Catalan healthcare; Decision-making; Multi-criteria decision analysis; Orphan drugs.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
DRUG EVALUATION AND DECISION MAKING IN CATALONIA: DEVELOPMENT AND VALIDATION OF A METHODOLOGICAL FRAMEWORK BASED ON MULTI-CRITERIA DECISION ANALYSIS (MCDA) FOR ORPHAN DRUGS.Int J Technol Assess Health Care. 2017 Jan;33(1):111-120. doi: 10.1017/S0266462317000149. Epub 2017 Apr 24. Int J Technol Assess Health Care. 2017. PMID: 28434413
-
MULTI-CRITERIA DECISION ANALYSIS AS A DECISION-SUPPORT TOOL FOR DRUG EVALUATION: A PILOT STUDY IN A PHARMACY AND THERAPEUTICS COMMITTEE SETTING.Int J Technol Assess Health Care. 2018 Jan;34(5):519-526. doi: 10.1017/S0266462318000569. Epub 2018 Oct 23. Int J Technol Assess Health Care. 2018. PMID: 30348241
-
Patient involvement in reflective multicriteria decision analysis to assist decision making in oncology.Int J Technol Assess Health Care. 2019 Jan;35(1):56-63. doi: 10.1017/S0266462318003641. Epub 2019 Feb 7. Int J Technol Assess Health Care. 2019. PMID: 30730288
-
International experiences in multicriteria decision analysis (MCDA) for evaluating orphan drugs: a scoping review.Expert Rev Pharmacoecon Outcomes Res. 2019 Aug;19(4):409-420. doi: 10.1080/14737167.2019.1633918. Epub 2019 Jul 1. Expert Rev Pharmacoecon Outcomes Res. 2019. PMID: 31210065
-
Identifying key unmet needs and value drivers in the treatment of focal-onset seizures (FOS) in patients with drug-resistant epilepsy (DRE) in Spain through Multi-Criteria Decision Analysis (MCDA).Epilepsy Behav. 2021 Sep;122:108222. doi: 10.1016/j.yebeh.2021.108222. Epub 2021 Aug 6. Epilepsy Behav. 2021. PMID: 34371462
Cited by
-
Integrative Review of Managed Entry Agreements: Chances and Limitations.Pharmacoeconomics. 2020 Nov;38(11):1165-1185. doi: 10.1007/s40273-020-00943-1. Pharmacoeconomics. 2020. PMID: 32734573 Review.
-
Dealing With Immunoglobulin Shortages: A Rationalization Plan From Evidence-Based and Data Collection.Front Public Health. 2022 May 19;10:893770. doi: 10.3389/fpubh.2022.893770. eCollection 2022. Front Public Health. 2022. PMID: 35664094 Free PMC article.
-
The value of the reflective discussion in decision-making using multi-criteria decision analysis (MCDA): an example of determining the value contribution of tabelecleucel for the treatment of the Epstein Barr virus-positive post-transplant lymphoproliferative disease (EBV+ PTLD).Orphanet J Rare Dis. 2024 Aug 23;19(1):308. doi: 10.1186/s13023-024-03324-5. Orphanet J Rare Dis. 2024. PMID: 39180132 Free PMC article.
-
Assessing the Preferences for Criteria in Multi-Criteria Decision Analysis in Treatments for Rare Diseases.Front Public Health. 2020 May 8;8:162. doi: 10.3389/fpubh.2020.00162. eCollection 2020. Front Public Health. 2020. PMID: 32457865 Free PMC article.
-
Description of the use of multicriteria to support pricing and reimbursement decisions by European health technology assessment bodies.BMC Health Serv Res. 2021 Aug 14;21(1):814. doi: 10.1186/s12913-021-06784-8. BMC Health Serv Res. 2021. PMID: 34391431 Free PMC article.
References
-
- Ministerio de Sanidad Servicios Sociales e Igualdad . [cited 2017 May 1] Available from: National Health System. Spain. https://www.msssi.gob.es/organizacion/ministerio/home.htm.
-
- Generalitat de Catalunya. Pla de Salut de Catalunya 2016-2020. [cited 2017 May 1] Available from: http://salutweb.gencat.cat/ca/el_departament/Pla_salut/pla-de-salut-2016....
-
- Agencia de Evaluación de Tecnologías Sanitarias (AETS). Instituto de Salud Carlos III - Ministerio de Economía y Competitividad. Criterios de financiación y reembolso de los medicamentos huérfanos. 2016.
-
- Goetghebeur MM, Wagner M, Khoury H, Levitt RJ, Erickson LJ, Rindress D. Bridging health technology assessment (HTA) and efficient health care decision making with multicriteria decision analysis (MCDA): applying the EVIDEM framework to medicines appraisal. Med Decis Mak. 2012;32(2):376–388. doi: 10.1177/0272989X11416870. - DOI - PubMed

