Altered gut microbiota and inflammatory cytokine responses in patients with Parkinson's disease
- PMID: 31248424
- PMCID: PMC6598278
- DOI: 10.1186/s12974-019-1528-y
Altered gut microbiota and inflammatory cytokine responses in patients with Parkinson's disease
Abstract
Objective: Emerging evidence suggests that gut microbiome composition alterations affect neurodegeneration through neuroinflammation in the pathogenesis of Parkinson's disease (PD). Here, we evaluate gut microbiota alterations and host cytokine responses in a population of Taiwanese patients with PD.
Methods: Fecal microbiota communities from 80 patients with PD and 77 age and gender-matched controls were assessed by sequencing the V3-V4 region of the 16S ribosomal RNA gene. Diet and comorbidities were controlled in the analyses. Plasma concentrations of IL-1β, IL-2, IL-4, IL-6, IL-13, IL-18, GM-CSF, IFNγ, and TNFα were measured by a multiplex immunoassay and relationships between microbiota, clinical characteristics, and cytokine levels were analyzed in the PD group. We further examined the cytokine changes associated with the altered gut microbiota seen in patients with PD in another independent cohort of 120 PD patients and 120 controls.
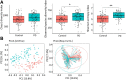
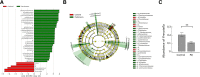
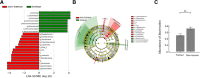
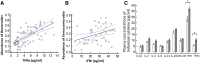
Results: Microbiota from patients with PD was altered relative to controls and dominated by Verrucomicrobia, Mucispirillum, Porphyromonas, Lactobacillus, and Parabacteroides. In contrast, Prevotella was more abundant in controls. The abundances of Bacteroides were more increased in patients with non-tremor PD subtype than patients with tremor subtype. Bacteroides abundance was correlated with motor symptom severity defined by UPDRS part III motor scores (rho = 0.637 [95% confidence interval 0.474 to 0.758], P < 0.01). Altered microbiota was correlated with plasma concentrations of IFNγ and TNFα. There was a correlation between Bacteroides and plasma level of TNFα (rho = 0.638 [95% CI: 0.102-0.887], P = 0.02); and a correlation between Verrucomicrobia abundance and plasma concentrations of IFNγ (rho = 0.545 [95% CI - 0.043-0.852], P = 0.05). The elevated plasma cytokine responses were confirmed in an additional independent 120 patients with PD and 120 controls (TNFα: PD vs. control 8.51 ± 4.63 pg/ml vs. 4.82 ± 2.23 pg/ml, P < 0.01; and IFNγ: PD vs. control: 38.45 ± 7.12 pg/ml vs. 32.79 ± 8.03 pg/ml, P = 0.03).
Conclusions: This study reveals altered gut microbiota in PD and its correlation with clinical phenotypes and severity in our population. The altered plasma cytokine profiles associated with gut microbiome composition alterations suggest aberrant immune responses may contribute to inflammatory processes in PD.
Keywords: Cytokines; Dysbiosis; Gut microbiome; Neuroinflammation; Parkinson’s disease.
Conflict of interest statement
All authors declare that they have no competing interests.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

