Clearance of apoptotic cells by mesenchymal stem cells contributes to immunosuppression via PGE2
- PMID: 31248835
- PMCID: PMC6642220
- DOI: 10.1016/j.ebiom.2019.06.016
Clearance of apoptotic cells by mesenchymal stem cells contributes to immunosuppression via PGE2
Abstract
Background: Defective clearance of apoptotic cells (ACs) has been suggested to be involved in the pathogenesis of systemic lupus erythematosus (SLE). Mesenchymal stem cells (MSCs) exhibit promising therapeutic effects on SLE, but whether MSCs phagocytose ACs and contributes to the underlying mechanism in the treatment of SLE remain unknown.
Methods: Human umbilical cord (UC) MSCs were co-cultured with ACs, and the engulfment of ACs by MSCs was either detected by flow cytometry or observed under confocal laser scanning microscope. Peripheral blood mononuclear cells (PBMCs) from healthy controls (HCs) were cultured in MSC conditioned medium (MCM) or MSC exposed to ACs (AC-MSC) conditioned medium (ACMCM), and then CD4+ T cell proliferation was detected. Soluble factors including prostaglandin (PG)E2 in the supernatants of MSCs and AC-MSCs, as well as in the mouse peritoneal lavage fluids (PLF) were determined by enzyme-linked immunosorbent assay (ELISA). Cyclooxygenase (COX)2 inhibitors and siRNA transfection were utilized to determine the function of COX2/PGE2 in AC-MSC-mediated immunosuppression. PGE2 metabolites (PGEM) in the plasma of SLE patients were measured before and 24 h after MSC transplantation respectively.
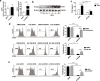
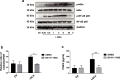
Findings: Human UC MSCs possessed the ability to engulf ACs. AC-MSCs increased MSC-mediated suppression of CD4+ T cell proliferation compared to MSCs alone. Mechanistically, ACs stimulated MSCs to express COX2 and consequently produced PGE2 that inhibited T cell responses. NF-κB signalling pathway mediated the activation of COX2/PGE2 in AC-MSCs. Importantly, in patients with SLE, the plasma PGEM levels increased significantly in those with reduced apoptotic mononuclear cells in peripheral blood after MSC transplantation.
Interpretation: Clearance of ACs by MSCs contributes to immunosuppressive function via increasing PGE2 production. These findings reveal a previously unrecognized role of MSC-mediated phagocytosis of ACs in MSC-based immunotherapy. FUND: This study was supported by grants from the Chinese Major International (Regional) Joint Research Project (No. 81720108020), the Jiangsu Province Major Research and Development Program (No. BE2015602) and the Jiangsu Province 333 Talent Grant (BRA2016001). WJ. Chen was supported by the Intramural Research Program of NIH, NIDCR.
Copyright © 2019. Published by Elsevier B.V.
Figures




References
-
- Tsokos G.C., Lo M.S., Costa Reis P., Sullivan K.E. New insights into the immunopathogenesis of systemic lupus erythematosus. Nat Rev Rheumatol. 2016;12:716–730. - PubMed
-
- Munoz L.E., Lauber K., Schiller M., Manfredi A.A., Herrmann M. The role of defective clearance of apoptotic cells in systemic autoimmunity. Nat Rev Rheumatol. 2010;6:280–289. - PubMed
-
- Shoshan Y., Mevorach D. Accelerated autoimmune disease in MRL/MpJ-Faslpr but not in MRL/MpJ following immunization with high load of syngeneic late apoptotic cells. Autoimmunity. 2004;37:103–109. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

