Addition of sirolimus to standard cyclosporine plus mycophenolate mofetil-based graft-versus-host disease prophylaxis for patients after unrelated non-myeloablative haemopoietic stem cell transplantation: a multicentre, randomised, phase 3 trial
- PMID: 31248843
- PMCID: PMC6686903
- DOI: 10.1016/S2352-3026(19)30088-2
Addition of sirolimus to standard cyclosporine plus mycophenolate mofetil-based graft-versus-host disease prophylaxis for patients after unrelated non-myeloablative haemopoietic stem cell transplantation: a multicentre, randomised, phase 3 trial
Abstract
Background: Acute graft-versus-host-disease (GVHD) after non-myeloablative human leucocyte antigen (HLA)-matched, unrelated donor, allogeneic haemopoietic stem cell transplantation (HSCT) is associated with considerable morbidity and mortality. This trial aimed to evaluate the efficacy of adding sirolimus to the standard cyclosporine and mycophenolate mofetil prophylaxis therapy for preventing acute GVHD in this setting.
Methods: This multicentre, randomised, phase 3 trial took place at nine HSCT centres based in the USA, Denmark, and Germany. Eligible patients were diagnosed with advanced haematological malignancies treatable by allogeneic HSCT, had a Karnofsky score greater than or equal to 60, were aged older than 50 years, or if they were aged 50 years or younger, were considered at high risk of regimen-related toxicity associated with a high-dose pre-transplantation conditioning regimen. Patients were randomly allocated by an adaptive randomisation scheme stratified by transplantation centre to receive either the standard GVHD prophylaxis regimen (cyclosporine and mycophenolate mofetil) or the triple-drug combination regimen (cyclosporine, mycophenolate mofetil, and sirolimus). Patients and physicians were not masked to treatment. All patients were prepared for HSCT with fludarabine (30 mg/m2 per day) 4, 3, and 2 days before receiving 2 or 3 Gy total body irradiation on the day of HSCT (day 0). In both study groups, 5·0 mg/kg of cyclosporine was administered orally twice daily starting 3 days before HSCT, and (in the absence of GVHD) tapered from day 96 through to day 150. In the standard GVHD prophylaxis group, 15 mg/kg of mycophenolate mofetil was given orally three times daily from day 0 until day 30, then twice daily until day 150, and (in the absence of GVHD) tapered off by day 180. In the triple-drug group, mycophenolate mofetil doses were the same as in the standard group, but the drug was discontinued on day 40. Sirolimus was started 3 days before HSCT, taken orally at 2 mg once daily and adjusted to maintain trough concentrations between 3-12 ng/mL through to day 150, and (in the absence of GVHD) tapered off by day 180. The primary endpoint was the cumulative incidence of grade 2-4 acute GVHD at day 100 post-transplantation. Secondary endpoints were non-relapse mortality, overall survival, progression-free survival, cumulative incidence of grade 3-4 acute GVHD, and cumulative incidence of chronic GVHD. Efficacy and safety analyses were per protocol, including all patients who received conditioning treatment and underwent transplantation. Toxic effects were measured according to the Common Terminology Criteria for Adverse Events (CTCAE). The current study was closed prematurely by recommendation of the Data and Safety Monitoring Board on July 27, 2016, after 168 patients received the allocated intervention, based on the results of a prespecified interim analysis for futility. This study is registered with ClinicalTrials.gov, number NCT01231412.
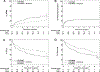
Findings: Participants were recruited between Nov 1, 2010, and July 27, 2016. Of 180 patients enrolled in the study, 167 received the complete study intervention and were included in safety and efficacy analyses: 77 patients in the standard GVHD prophylaxis group and 90 in the triple-drug group. At the time of analysis, median follow-up was 48 months (IQR 31-60). The cumulative incidence of grade 2-4 acute GVHD at day 100 was lower in the triple-drug group compared with the standard GVHD prophylaxis group (26% [95% CI 17-35] in the triple-drug group vs 52% [41-63] in the standard group; HR 0·45 [95% CI 0·28-0·73]; p=0·0013). After 1 and 4 years, non-relapse mortality increased to 4% (95% CI 0-9) and 16% (8-24) in the triple-drug group and 16% (8-24) and 32% (21-43) in the standard group (HR 0·48 [0·26-0·90]; p=0·021). Overall survival at 1 year was 86% (95% CI 78-93) in the triple-drug group and 70% in the standard group (60-80) and at 4 years it was 64% in the triple-drug group (54-75) and 46% in the standard group (34-57%; HR 0·62 [0·40-0·97]; p=0·035). Progression-free survival at 1 year was 77% (95% CI 68-85) in the triple-drug group and 64% (53-74) in the standard drug group, and at 4 years it was 59% in the triple-drug group (49-70) and 41% in the standard group (30-53%; HR 0·64 [0·42-0·99]; p=0·045). We observed no difference in the cumulative incidence of grade 3-4 acute GVHD (2% [0-5] in the triple-drug group vs 8% [2-14] in the standard group; HR 0·55 [0·16-1·96]; p=0·36) and chronic GVHD (49% [39-59] in triple-drug group vs 50% [39-61] in the standard group; HR 0·94 [0·62-1·40]; p=0·74). In both groups the most common CTCAE grade 4 or higher toxic effects were pulmonary.
Interpretation: Adding sirolimus to cyclosporine and mycophenolate mofetil resulted in a significantly lower proportion of patients developing acute GVHD compared with patients treated with cyclosporine and mycophenolate mofetil alone. Based on these results, the combination of cyclosporine, mycophenolate mofetil, and sirolimus has become the new standard GVHD prophylaxis regimen for patients treated with non-myeloablative conditioning and HLA-matched unrelated HSCT at the Fred Hutchinson Cancer Research Center.
Funding: National Institutes of Health.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests
The authors have no conflicts of interest to declare.
Figures


Comment in
-
Sirolimus-based graft-versus-host disease prophylaxis.Lancet Haematol. 2019 Aug;6(8):e387-e388. doi: 10.1016/S2352-3026(19)30108-5. Epub 2019 Jun 24. Lancet Haematol. 2019. PMID: 31248842 No abstract available.
Similar articles
-
Addition of anti-thymocyte globulin to standard graft-versus-host disease prophylaxis versus standard treatment alone in patients with haematological malignancies undergoing transplantation from unrelated donors: final analysis of a randomised, open-label, multicentre, phase 3 trial.Lancet Haematol. 2020 Feb;7(2):e100-e111. doi: 10.1016/S2352-3026(19)30220-0. Epub 2020 Jan 17. Lancet Haematol. 2020. PMID: 31958417 Clinical Trial.
-
Triple-Drug Graft-versus-Host Disease Prophylaxis after HLA-Matched Unrelated Donor Nonmyeloablative Allogenic Hematopoietic Stem Cell Transplantation.Transplant Cell Ther. 2023 Sep;29(9):575.e1-575.e6. doi: 10.1016/j.jtct.2023.05.022. Epub 2023 Jun 9. Transplant Cell Ther. 2023. PMID: 37301257 Clinical Trial.
-
Efficacy of two different doses of rabbit anti-T-lymphocyte globulin to prevent graft-versus-host disease in children with haematological malignancies transplanted from an unrelated donor: a multicentre, randomised, open-label, phase 3 trial.Lancet Oncol. 2017 Aug;18(8):1126-1136. doi: 10.1016/S1470-2045(17)30417-5. Epub 2017 Jul 10. Lancet Oncol. 2017. PMID: 28705454 Clinical Trial.
-
Comparison of Tacrolimus and Cyclosporine Combined With Methotrexate for Graft Versus Host Disease Prophylaxis After Allogeneic Hematopoietic Cell Transplantation.Transplantation. 2020 Feb;104(2):428-436. doi: 10.1097/TP.0000000000002836. Transplantation. 2020. PMID: 31283681
-
Hematopoietic stem cell transplantation in serine/threonine kinase 4 (STK4) deficiency: Report of two cases and literature review.Pediatr Transplant. 2023 Mar;27(2):e14439. doi: 10.1111/petr.14439. Epub 2022 Nov 16. Pediatr Transplant. 2023. PMID: 36394186 Review.
Cited by
-
Double Malignancy and Double Transplant-A Bumpy Road to Success.Medicina (Kaunas). 2023 Jun 27;59(7):1209. doi: 10.3390/medicina59071209. Medicina (Kaunas). 2023. PMID: 37512021 Free PMC article.
-
GvHD prophylaxis with tacrolimus, sirolimus, and mycophenolate mofetil after reduced intensity conditioning hematopoietic stem cell allogeneic transplantation.Bone Marrow Transplant. 2025 Jun;60(6):832-840. doi: 10.1038/s41409-025-02562-w. Epub 2025 Apr 8. Bone Marrow Transplant. 2025. PMID: 40200003 Free PMC article.
-
Current Status and Perspectives of Allogeneic Hematopoietic Stem Cell Transplantation in Elderly Patients with Acute Myeloid Leukemia.Stem Cells Transl Med. 2022 May 27;11(5):461-477. doi: 10.1093/stcltm/szac015. Stem Cells Transl Med. 2022. PMID: 35438781 Free PMC article. Review.
-
Sirolimus as Rescue Therapy for Refractory/Relapsed Immune Thrombocytopenia: Results of a Single-Center, Prospective, Single-Arm Study.Front Med (Lausanne). 2020 Mar 31;7:110. doi: 10.3389/fmed.2020.00110. eCollection 2020. Front Med (Lausanne). 2020. PMID: 32296709 Free PMC article.
-
GVHD Prophylaxis 2020.Front Immunol. 2021 Apr 7;12:605726. doi: 10.3389/fimmu.2021.605726. eCollection 2021. Front Immunol. 2021. PMID: 33897681 Free PMC article. Review.
References
-
- McSweeney PA, Niederwieser D, Shizuru JA, et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood 2001; 97(11): 3390–400. - PubMed
-
- Niederwieser D, Maris M, Shizuru JA, et al. Low-dose total body irradiation (TBI) and fludarabine followed by hematopoietic cell transplantation (HCT) from HLA-matched or mismatched unrelated donors and postgrafting immunosuppression with cyclosporine and mycophenolate mofetil (MMF) can induce durable complete chimerism and sustained remissions in patients with hematological diseases. Blood 2003; 101(4): 1620–9. - PubMed
-
- Efron B Forcing a sequential experiment to be balanced. Biometrika 1971; 58(3): 403–17.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

