Considering the methodological limitations in the evidence base of antidepressants for depression: a reanalysis of a network meta-analysis
- PMID: 31248914
- PMCID: PMC6597641
- DOI: 10.1136/bmjopen-2018-024886
Considering the methodological limitations in the evidence base of antidepressants for depression: a reanalysis of a network meta-analysis
Abstract
Objectives: To investigate whether the conclusion of a recent systematic review and network meta-analysis (Cipriani et al) that antidepressants are more efficacious than placebo for adult depression was supported by the evidence.
Design: Reanalysis of a systematic review, with meta-analyses.
Data sources: 522 trials (116 477 participants) as reported in the systematic review by Cipriani et al and clinical study reports for 19 of these trials.
Analysis: We used the Cochrane Handbook's risk of bias tool and the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach to evaluate the risk of bias and the certainty of evidence, respectively. The impact of several study characteristics and publication status was estimated using pairwise subgroup meta-analyses.
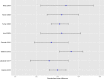
Results: Several methodological limitations in the evidence base of antidepressants were either unrecognised or underestimated in the systematic review by Cipriani et al. The effect size for antidepressants versus placebo on investigator-rated depression symptom scales was higher in trials with a 'placebo run-in' study design compared with trials without a placebo run-in design (p=0.05). The effect size of antidepressants was higher in published trials compared with unpublished trials (p<0.0001). The outcome data reported by Cipriani et al differed from the clinical study reports in 12 (63%) of 19 trials. The certainty of the evidence for the placebo-controlled comparisons should be very low according to GRADE due to a high risk of bias, indirectness of the evidence and publication bias. The mean difference between antidepressants and placebo on the 17-item Hamilton depression rating scale (range 0-52 points) was 1.97 points (95% CI 1.74 to 2.21).
Conclusions: The evidence does not support definitive conclusions regarding the benefits of antidepressants for depression in adults. It is unclear whether antidepressants are more efficacious than placebo.
Keywords: adult psychiatry.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
Pharmacological and electronic cigarette interventions for smoking cessation in adults: component network meta-analyses.Cochrane Database Syst Rev. 2023 Sep 12;9(9):CD015226. doi: 10.1002/14651858.CD015226.pub2. Cochrane Database Syst Rev. 2023. PMID: 37696529 Free PMC article.
-
Prenatal administration of progestogens for preventing spontaneous preterm birth in women with a multiple pregnancy.Cochrane Database Syst Rev. 2019 Nov 20;2019(11):CD012024. doi: 10.1002/14651858.CD012024.pub3. Cochrane Database Syst Rev. 2019. PMID: 31745984 Free PMC article.
-
Assessing the comparative effects of interventions in COPD: a tutorial on network meta-analysis for clinicians.Respir Res. 2024 Dec 21;25(1):438. doi: 10.1186/s12931-024-03056-x. Respir Res. 2024. PMID: 39709425 Free PMC article. Review.
-
Prevalence and odds of anxiety and depression in cutaneous malignant melanoma: a proportional meta-analysis and regression.Br J Dermatol. 2024 Jun 20;191(1):24-35. doi: 10.1093/bjd/ljae011. Br J Dermatol. 2024. PMID: 38197404
-
Janus Kinase Inhibitors for Alopecia Areata: A Systematic Review and Meta-Analysis.JAMA Netw Open. 2023 Jun 1;6(6):e2320351. doi: 10.1001/jamanetworkopen.2023.20351. JAMA Netw Open. 2023. PMID: 37368402 Free PMC article.
Cited by
-
Patient perspectives and experiences with psilocybin treatment for treatment-resistant depression: a qualitative study.Sci Rep. 2024 Feb 5;14(1):2929. doi: 10.1038/s41598-024-53188-9. Sci Rep. 2024. PMID: 38316896 Free PMC article. Clinical Trial.
-
Individual response to antidepressants for depression in adults-a meta-analysis and simulation study.PLoS One. 2020 Aug 27;15(8):e0237950. doi: 10.1371/journal.pone.0237950. eCollection 2020. PLoS One. 2020. PMID: 32853222 Free PMC article.
-
Guidelines for the pharmacological acute treatment of major depression: conflicts with current evidence as demonstrated with the German S3-guidelines.BMC Psychiatry. 2019 Sep 2;19(1):265. doi: 10.1186/s12888-019-2230-4. BMC Psychiatry. 2019. PMID: 31477074 Free PMC article.
-
Aerobic Exercise Training and Depressive Symptoms in People With Multiple Sclerosis: Brief Report on Default-Mode Network Resting-State Functional Connectivity.Int J MS Care. 2025 Feb 3;27(Q1):34-41. doi: 10.7224/1537-2073.2024-003. eCollection 2025 Jan. Int J MS Care. 2025. PMID: 39906605 Free PMC article.
-
Workplace-Related Interpersonal Group Psychotherapy to Improve Life at Work in Individuals With Major Depressive Disorders: A Randomized Interventional Pilot Study.Front Psychiatry. 2020 Mar 17;11:168. doi: 10.3389/fpsyt.2020.00168. eCollection 2020. Front Psychiatry. 2020. PMID: 32256402 Free PMC article.
References
-
- World Health Organisation, Media Centre. Depression: Fact Sheet. 2017. http://www.who.int/mediacentre/factsheets/fs369/en/ (accessed 1 May 2018).
-
- Danish Health Authority. MedicinForbrug - Indblik 2017: Laveste antal brugere af antidepressiv medicin de seneste 10 år [Medicine usage - 2017: Lowest number of antidepressant users in the last 10 years]. 2017. https://sundhedsdatastyrelsen.dk/-/media/sds/filer/find-tal-og-analyser/... (accessed 1 May 2018).
-
- U.S. Department of Health and Human Services: Centers for Disease Control and Prevention. 2017. Antidepressant Use Among Persons Aged 12 and Over: United States, 2011–2014 Available from: accessed 1 May 2018.
-
- The Guardian. NHS prescribed record number of antidepressants last year [updated 29 June 2017] Available from: www.theguardian.com/society/2017/jun/29/nhs-prescribed-record-number-of-... 10.1111/bdi.12609 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
