Efficacy and Safety of Esaxerenone (CS-3150) for the Treatment of Type 2 Diabetes with Microalbuminuria: A Randomized, Double-Blind, Placebo-Controlled, Phase II Trial
- PMID: 31248950
- PMCID: PMC6682830
- DOI: 10.2215/CJN.14751218
Efficacy and Safety of Esaxerenone (CS-3150) for the Treatment of Type 2 Diabetes with Microalbuminuria: A Randomized, Double-Blind, Placebo-Controlled, Phase II Trial
Abstract
Background and objectives: The progression of kidney disease in some patients with type 2 diabetes mellitus may not be adequately suppressed by renin-angiotensin system inhibitors. Esaxerenone (CS-3150) is a nonsteroidal mineralocorticoid receptor blocker that has shown kidney protective effects in preclinical studies, and it is a potential add-on therapy to treat diabetic kidney disease. This phase 2 study evaluated the efficacy and safety of esaxerenone in Japanese patients with type 2 diabetes mellitus and microalbuminuria.
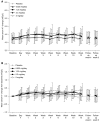
Design, setting, participants, & measurements: This multicenter, randomized, double-blind, placebo-controlled trial enrolled 365 hypertensive or normotensive patients with type 2 diabetes mellitus and microalbuminuria (urinary albumin-to-creatinine ratio ≥45 to <300 mg/g creatinine) treated with renin-angiotensin system inhibitor who had eGFR≥30 ml/min per 1.73 m2. Participants were randomized to receive 0.625, 1.25, 2.5, or 5 mg/d esaxerenone or placebo for 12 weeks. The primary end point was the change in urinary albumin-to-creatinine ratio from baseline to week 12 (with last observation carried forward).
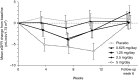
Results: Esaxerenone treatment at 1.25, 2.5, and 5 mg/d significantly reduced urinary albumin-to-creatinine ratio by the end of treatment (38%, 50%, and 56%, respectively) compared with placebo (7%; all P<0.001). The urinary albumin-to-creatinine ratio remission rate (defined as urinary albumin-to-creatinine ratio <30 mg/g creatinine at the end of treatment and ≥30% decrease from baseline) was 21% in the 2.5- and 5-mg/d groups versus 3% for placebo (both P<0.05). Adverse events occurred slightly more frequently with esaxerenone versus placebo, but the frequencies of drug-related adverse events and discontinuation rates were similar in the placebo and the 0.625-, 1.25-, and 2.5-mg/d groups. Drug-related adverse events and treatment discontinuations were marginally higher in the 5-mg/d group. The most common drug-related adverse event was hyperkalemia, which was dose proportional.
Conclusions: Adding esaxerenone at 1.25, 2.5, and 5 mg/d for 12 weeks to an ongoing renin-angiotensin system inhibitor significantly reduces urinary albumin-to-creatinine ratio in patients with type 2 diabetes mellitus and microalbuminuria.
Keywords: Esaxerenone; Urine albumin-to-creatinine ratio; microalbuminuria; mineralocorticoid receptor antagonist; placebo-controlled; randomized; remission; type 2 diabetes mellitus.
Copyright © 2019 by the American Society of Nephrology.
Figures



References
-
- Molitch ME, DeFronzo RA, Franz MJ, Keane WF, Mogensen CE, Parving HH, Steffes MW; American Diabetes Association : Nephropathy in diabetes. Diabetes Care 27[Suppl 1]: S79–S83, 2004 - PubMed
-
- Japanese Society of Nephrology : Evidence-based clinical practice guideline for CKD (2013). Clin Exp Nephrol 18: 346–423, 2014
-
- Shimamoto K, Ando K, Fujita T, Hasebe N, Higaki J, Horiuchi M, Imai Y, Imaizumi T, Ishimitsu T, Ito M, Ito S, Itoh H, Iwao H, Kai H, Kario K, Kashihara N, Kawano Y, Kim-Mitsuyama S, Kimura G, Kohara K, Komuro I, Kumagai H, Matsuura H, Miura K, Morishita R, Naruse M, Node K, Ohya Y, Rakugi H, Saito I, Saitoh S, Shimada K, Shimosawa T, Suzuki H, Tamura K, Tanahashi N, Tsuchihashi T, Uchiyama M, Ueda S, Umemura S; Japanese Society of Hypertension Committee for Guidelines for the Management of Hypertension : The Japanese society of hypertension guidelines for the management of hypertension (JSH 2014). Hypertens Res 37: 253–390, 2014 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

