Ca2+ flux through splice variants of the ATP-gated ionotropic receptor P2X7 is regulated by its cytoplasmic N terminus
- PMID: 31248985
- PMCID: PMC6699846
- DOI: 10.1074/jbc.RA119.009666
Ca2+ flux through splice variants of the ATP-gated ionotropic receptor P2X7 is regulated by its cytoplasmic N terminus
Abstract
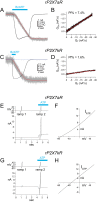
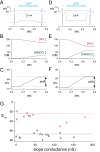
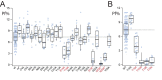
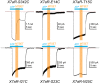
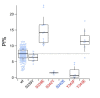
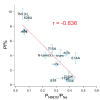
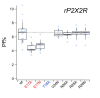
Activation of ionotropic P2X receptors increases free intracellular Ca2+ ([Ca2+] i ) by initiating a transmembrane cation flux. We studied the "a" and "k" splice variants of the rat purinergic P2X7 receptor (rP2X7aR and rP2X7kR) to exhibit a significant difference in Ca2+ flux through this channel. This difference is surprising because the variants share absolute sequence identity in the area of the pore that defines ionic selectivity. Here, we used patch-clamp fluorometry and chimeric receptors to show that the fraction of the total current carried by Ca2+ is a function of the primary sequence of the cytoplasmic N terminus. Using scanning mutagenesis, we identified five sites within the N terminus that respond to mutagenesis with a decrease in fractional calcium current and an increase in permeability to the polyatomic cation, N-methyl-d-glucamine (NMDG+), relative to Na+ (PNMDG/PNa). We tested the hypothesis that these sites line the permeation pathway by measuring the ability of thiol-reactive MTSET+ to alter the current of cysteine-substituted variants, but we detected no effect. Finally, we studied the homologous sites of the rat P2X2 receptor (rP2X2R) and observed that substitutions at Glu17 significantly reduced the fractional calcium current. Taken together, our results suggest that a change in the structure of the N terminus alters the ability of an intra-pore Ca2+ selectivity filter to discriminate among permeating cations. These results are noteworthy for two reasons: they identify a previously unknown outcome of mutagenesis of the N-terminal domain, and they suggest caution when assigning structure to function for truncated P2X receptors that lack a part of the N terminus.
Keywords: P2X 7 (P2X7) P2RX7; calcium channel; calcium transport; fractional calcium current; ion channel; ligand-gated; patch clamp; patch-clamp fluorometry; permeability; purinergic receptor; splice variant.
© 2019 Liang et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures

Similar articles
-
On the role of the first transmembrane domain in cation permeability and flux of the ATP-gated P2X2 receptor.J Biol Chem. 2008 Feb 22;283(8):5110-7. doi: 10.1074/jbc.M708713200. Epub 2007 Nov 29. J Biol Chem. 2008. PMID: 18048351
-
Quantifying Ca2+ current and permeability in ATP-gated P2X7 receptors.J Biol Chem. 2015 Mar 20;290(12):7930-42. doi: 10.1074/jbc.M114.627810. Epub 2015 Feb 2. J Biol Chem. 2015. PMID: 25645917 Free PMC article.
-
Rectification of ATP-gated current of rat P2X2 and P2X7 receptors depends on the cytoplasmic N-terminus.Biochem Biophys Res Commun. 2023 Dec 25;688:149213. doi: 10.1016/j.bbrc.2023.149213. Epub 2023 Nov 7. Biochem Biophys Res Commun. 2023. PMID: 37976814
-
Human P2X7 receptors - Properties of single ATP-gated ion channels.Biochem Pharmacol. 2021 May;187:114307. doi: 10.1016/j.bcp.2020.114307. Epub 2020 Oct 29. Biochem Pharmacol. 2021. PMID: 33130127 Review.
-
P2X7 Variants in Pathophysiology.Int J Mol Sci. 2024 Jun 18;25(12):6673. doi: 10.3390/ijms25126673. Int J Mol Sci. 2024. PMID: 38928378 Free PMC article. Review.
Cited by
-
P2X7 in Cancer: From Molecular Mechanisms to Therapeutics.Front Pharmacol. 2020 Jun 4;11:793. doi: 10.3389/fphar.2020.00793. eCollection 2020. Front Pharmacol. 2020. PMID: 32581786 Free PMC article. Review.
-
ATP-gated P2X7 receptor as a potential target for prostate cancer.Hum Cell. 2022 Sep;35(5):1346-1354. doi: 10.1007/s13577-022-00729-x. Epub 2022 Jun 3. Hum Cell. 2022. PMID: 35657562 Review.
-
Using Whole-Cell Electrophysiology and Patch-Clamp Photometry to Characterize P2X7 Receptor Currents.Methods Mol Biol. 2022;2510:217-237. doi: 10.1007/978-1-0716-2384-8_11. Methods Mol Biol. 2022. PMID: 35776327
-
A genetic correlation and bivariate genome-wide association study of grip strength and depression.PLoS One. 2022 Dec 15;17(12):e0278392. doi: 10.1371/journal.pone.0278392. eCollection 2022. PLoS One. 2022. PMID: 36520780 Free PMC article.
-
Functional role of P2X7 purinergic receptor in cancer and cancer-related pain.Purinergic Signal. 2024 May 21. doi: 10.1007/s11302-024-10019-w. Online ahead of print. Purinergic Signal. 2024. PMID: 38771429 Review.
References
-
- Nicke A., Grutter T., and Egan T. M. (2018) in The Oxford Handbook of Neuronal Ion Channels (Bhattacharjee A., ed) pp. 1–32, Oxford University Press, Oxford, UK
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

