Ca2+ flux through splice variants of the ATP-gated ionotropic receptor P2X7 is regulated by its cytoplasmic N terminus
- PMID: 31248985
- PMCID: PMC6699846
- DOI: 10.1074/jbc.RA119.009666
Ca2+ flux through splice variants of the ATP-gated ionotropic receptor P2X7 is regulated by its cytoplasmic N terminus
Abstract
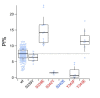
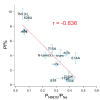
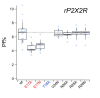
Activation of ionotropic P2X receptors increases free intracellular Ca2+ ([Ca2+] i ) by initiating a transmembrane cation flux. We studied the "a" and "k" splice variants of the rat purinergic P2X7 receptor (rP2X7aR and rP2X7kR) to exhibit a significant difference in Ca2+ flux through this channel. This difference is surprising because the variants share absolute sequence identity in the area of the pore that defines ionic selectivity. Here, we used patch-clamp fluorometry and chimeric receptors to show that the fraction of the total current carried by Ca2+ is a function of the primary sequence of the cytoplasmic N terminus. Using scanning mutagenesis, we identified five sites within the N terminus that respond to mutagenesis with a decrease in fractional calcium current and an increase in permeability to the polyatomic cation, N-methyl-d-glucamine (NMDG+), relative to Na+ (PNMDG/PNa). We tested the hypothesis that these sites line the permeation pathway by measuring the ability of thiol-reactive MTSET+ to alter the current of cysteine-substituted variants, but we detected no effect. Finally, we studied the homologous sites of the rat P2X2 receptor (rP2X2R) and observed that substitutions at Glu17 significantly reduced the fractional calcium current. Taken together, our results suggest that a change in the structure of the N terminus alters the ability of an intra-pore Ca2+ selectivity filter to discriminate among permeating cations. These results are noteworthy for two reasons: they identify a previously unknown outcome of mutagenesis of the N-terminal domain, and they suggest caution when assigning structure to function for truncated P2X receptors that lack a part of the N terminus.
Keywords: P2X 7 (P2X7) P2RX7; calcium channel; calcium transport; fractional calcium current; ion channel; ligand-gated; patch clamp; patch-clamp fluorometry; permeability; purinergic receptor; splice variant.
© 2019 Liang et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures

References
-
- Nicke A., Grutter T., and Egan T. M. (2018) in The Oxford Handbook of Neuronal Ion Channels (Bhattacharjee A., ed) pp. 1–32, Oxford University Press, Oxford, UK
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

