An extracellular acidic cleft confers profound H+-sensitivity to epithelial sodium channels containing the δ-subunit in Xenopus laevis
- PMID: 31248986
- PMCID: PMC6699844
- DOI: 10.1074/jbc.RA119.008255
An extracellular acidic cleft confers profound H+-sensitivity to epithelial sodium channels containing the δ-subunit in Xenopus laevis
Abstract
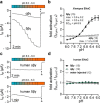
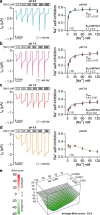
The limited sodium availability of freshwater and terrestrial environments was a major physiological challenge during vertebrate evolution. The epithelial sodium channel (ENaC) is present in the apical membrane of sodium-absorbing vertebrate epithelia and evolved as part of a machinery for efficient sodium conservation. ENaC belongs to the degenerin/ENaC protein family and is the only member that opens without an external stimulus. We hypothesized that ENaC evolved from a proton-activated sodium channel present in ionocytes of freshwater vertebrates and therefore investigated whether such ancestral traits are present in ENaC isoforms of the aquatic pipid frog Xenopus laevis Using whole-cell and single-channel electrophysiology of Xenopus oocytes expressing ENaC isoforms assembled from αβγ- or δβγ-subunit combinations, we demonstrate that Xenopus δβγ-ENaC is profoundly activated by extracellular acidification within biologically relevant ranges (pH 8.0-6.0). This effect was not observed in Xenopus αβγ-ENaC or human ENaC orthologs. We show that protons interfere with allosteric ENaC inhibition by extracellular sodium ions, thereby increasing the probability of channel opening. Using homology modeling of ENaC structure and site-directed mutagenesis, we identified a cleft region within the extracellular loop of the δ-subunit that contains several acidic amino acid residues that confer proton-sensitivity and enable allosteric inhibition by extracellular sodium ions. We propose that Xenopus δβγ-ENaC can serve as a model for investigating ENaC transformation from a proton-activated toward a constitutively-active ion channel. Such transformation might have occurred during the evolution of tetrapod vertebrates to enable bulk sodium absorption during the water-to-land transition.
Keywords: Xenopus; allosteric regulation; delta-subunit; epithelial sodium channel (ENaC); evolution; molecular evolution; pH; sodium self-inhibition; tetrapod; water–to–land transition.
© 2019 Wichmann et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article.
Figures







Similar articles
-
Incorporation of the δ-subunit into the epithelial sodium channel (ENaC) generates protease-resistant ENaCs in Xenopus laevis.J Biol Chem. 2018 May 4;293(18):6647-6658. doi: 10.1074/jbc.RA118.002543. Epub 2018 Mar 25. J Biol Chem. 2018. PMID: 29576549 Free PMC article.
-
The delta-subunit of the epithelial sodium channel (ENaC) enhances channel activity and alters proteolytic ENaC activation.J Biol Chem. 2009 Oct 16;284(42):29024-40. doi: 10.1074/jbc.M109.018945. Epub 2009 Aug 28. J Biol Chem. 2009. PMID: 19717556 Free PMC article.
-
δβγ-ENaC is inhibited by CFTR but stimulated by cAMP in Xenopus laevis oocytes.Am J Physiol Lung Cell Mol Physiol. 2017 Feb 1;312(2):L277-L287. doi: 10.1152/ajplung.00375.2016. Epub 2016 Dec 9. Am J Physiol Lung Cell Mol Physiol. 2017. PMID: 27941075
-
Evolution of epithelial sodium channels: current concepts and hypotheses.Am J Physiol Regul Integr Comp Physiol. 2020 Oct 1;319(4):R387-R400. doi: 10.1152/ajpregu.00144.2020. Epub 2020 Aug 12. Am J Physiol Regul Integr Comp Physiol. 2020. PMID: 32783689 Review.
-
ASIC and ENaC type sodium channels: conformational states and the structures of the ion selectivity filters.FEBS J. 2017 Feb;284(4):525-545. doi: 10.1111/febs.13840. Epub 2016 Sep 15. FEBS J. 2017. PMID: 27580245 Review.
Cited by
-
A novel homozygous mutation (p.N958K) of SLC12A3 in Gitelman syndrome is associated with endoplasmic reticulum stress.J Endocrinol Invest. 2021 Mar;44(3):471-480. doi: 10.1007/s40618-020-01329-y. Epub 2020 Jul 8. J Endocrinol Invest. 2021. PMID: 32642858
-
Varying Selection Pressure for a Na+ Sensing Site in Epithelial Na+ Channel Subunits Reflect Divergent Roles in Na+ Homeostasis.Mol Biol Evol. 2024 Aug 2;41(8):msae162. doi: 10.1093/molbev/msae162. Mol Biol Evol. 2024. PMID: 39101592 Free PMC article.
-
The evolutionary path of the epithelial sodium channel δ-subunit in Cetartiodactyla points to a role in sodium sensing.Commun Biol. 2025 Jul 4;8(1):1004. doi: 10.1038/s42003-025-08436-7. Commun Biol. 2025. PMID: 40615540 Free PMC article.
-
Structural insights into subunit-dependent functional regulation in epithelial sodium channels.Structure. 2025 Feb 6;33(2):349-362.e4. doi: 10.1016/j.str.2024.11.013. Epub 2024 Dec 11. Structure. 2025. PMID: 39667931
-
Accessibility of ENaC extracellular domain central core residues.J Biol Chem. 2022 May;298(5):101860. doi: 10.1016/j.jbc.2022.101860. Epub 2022 Mar 23. J Biol Chem. 2022. PMID: 35339489 Free PMC article.
References
-
- Kerem E., Bistritzer T., Hanukoglu A., Hofmann T., Zhou Z., Bennett W., MacLaughlin E., Barker P., Nash M., Quittell L., Boucher R., and Knowles M. R. (1999) Pulmonary epithelial sodium-channel dysfunction and excess airway liquid in pseudohypoaldosteronism. N. Engl. J. Med. 341, 156–162 10.1056/NEJM199907153410304 - DOI - PubMed
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources

