Direct observation of intermediates in the SufS cysteine desulfurase reaction reveals functional roles of conserved active-site residues
- PMID: 31248989
- PMCID: PMC6699847
- DOI: 10.1074/jbc.RA119.009471
Direct observation of intermediates in the SufS cysteine desulfurase reaction reveals functional roles of conserved active-site residues
Abstract
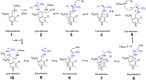
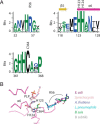
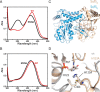
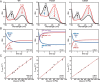
Iron-sulfur (Fe-S) clusters are necessary for the proper functioning of numerous metalloproteins. Fe-S cluster (Isc) and sulfur utilization factor (Suf) pathways are the key biosynthetic routes responsible for generating these Fe-S cluster prosthetic groups in Escherichia coli Although Isc dominates under normal conditions, Suf takes over during periods of iron depletion and oxidative stress. Sulfur acquisition via these systems relies on the ability to remove sulfur from free cysteine using a cysteine desulfurase mechanism. In the Suf pathway, the dimeric SufS protein uses the cofactor pyridoxal 5'-phosphate (PLP) to abstract sulfur from free cysteine, resulting in the production of alanine and persulfide. Despite much progress, the stepwise mechanism by which this PLP-dependent enzyme operates remains unclear. Here, using rapid-mixing kinetics in conjunction with X-ray crystallography, we analyzed the pre-steady-state kinetics of this process while assigning early intermediates of the mechanism. We employed H123A and C364A SufS variants to trap Cys-aldimine and Cys-ketimine intermediates of the cysteine desulfurase reaction, enabling direct observations of these intermediates and associated conformational changes of the SufS active site. Of note, we propose that Cys-364 is essential for positioning the Cys-aldimine for Cα deprotonation, His-123 acts to protonate the Ala-enamine intermediate, and Arg-56 facilitates catalysis by hydrogen bonding with the sulfhydryl of Cys-aldimine. Our results, along with previous SufS structural findings, suggest a detailed model of the SufS-catalyzed reaction from Cys binding to C-S bond cleavage and indicate that Arg-56, His-123, and Cys-364 are critical SufS residues in this C-S bond cleavage pathway.
Keywords: PLP; PLP-dependent sulfur abstraction; SufS; X-ray crystallography; cysteine desulfurase; cysteine sulfur bond cleavage; enzyme catalysis; enzyme mechanism; iron-sulfur protein; pre-steady-state kinetics.
© 2019 Blahut et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures



References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

