Evaluation of Candida peritonitis with underlying peritoneal fibrosis and efficacy of micafungin in murine models of intra-abdominal candidiasis
- PMID: 31249356
- PMCID: PMC6597535
- DOI: 10.1038/s41598-019-45776-x
Evaluation of Candida peritonitis with underlying peritoneal fibrosis and efficacy of micafungin in murine models of intra-abdominal candidiasis
Abstract
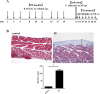
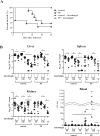
Candida peritonitis is a crucial disease, however the optimal antifungal therapy regimen has not been clearly defined. Peritoneal fibrosis (PF) can be caused by abdominal surgery, intra-abdominal infection, and malignant diseases, and is also widely recognized as a crucial complication of long-term peritoneal dialysis. However, the influence of PF on Candida peritonitis prognosis remains unknown. Here, we evaluated the severity of Candida peritonitis within the context of PF and the efficacy of micafungin using mice. A PF mouse model was generated by intraperitoneally administering chlorhexidine gluconate. Candida peritonitis, induced by intraperitoneal inoculation of Candida albicans, was treated with a 7-day consecutive subcutaneous administration of micafungin. Candida infection caused a higher mortality rate in the PF mice compared with the control mice on day 7. Proliferative Candida invasion into the peritoneum and intra-abdominal organs was confirmed pathologically only in the PF mice. However, all mice in both groups treated with micafungin survived until day 20. Micafungin treatment tends to suppress inflammatory cytokines in the plasma 12 h after infection in both groups. Our results suggest that PF enhances early mortality in Candida peritonitis. Prompt initiation and sufficient doses of micafungin had good efficacy for Candida peritonitis, irrespective of the underlying PF.
Conflict of interest statement
T.M. has received research grants from Astellas, Pfizer, MSD, and Asahi Kasei; K. Yamamoto and T.N. from MSD; K.I. and H.M. from Astellas, Pfizer, MSD, Sumitomo Dainippon, and Asahi Kasei; K. Yanagihara and S.K. from Astellas, Pfizer, MSD, Sumitomo Dainippon, Asahi Kasei, and Janssen. The remaining authors have no potential conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Figures


Similar articles
-
Comparison of Killing Activity of Micafungin Against Six Candida Species Isolated from Peritoneal and Pleural Cavities in RPMI-1640, 10 and 30% Serum.Mycopathologia. 2018 Dec;183(6):905-912. doi: 10.1007/s11046-018-0302-5. Epub 2018 Oct 31. Mycopathologia. 2018. PMID: 30382508
-
Plasma and peritoneal fluid population pharmacokinetics of micafungin in post-surgical patients with severe peritonitis.J Antimicrob Chemother. 2015 Oct;70(10):2854-61. doi: 10.1093/jac/dkv173. Epub 2015 Jul 14. J Antimicrob Chemother. 2015. PMID: 26180134
-
[Successful treatment with micafungin (MCFG) of severe peritonitis due to Candida parapsilosis with chronic renal failure patient on hemodialysis].Kansenshogaku Zasshi. 2005 Mar;79(3):195-200. doi: 10.11150/kansenshogakuzasshi1970.79.195. Kansenshogaku Zasshi. 2005. PMID: 15977561 Japanese.
-
[Antifungal activity and clinical efficacy of micafungin (funguard)].Nihon Ishinkin Gakkai Zasshi. 2005;46(4):217-22. doi: 10.3314/jjmm.46.217. Nihon Ishinkin Gakkai Zasshi. 2005. PMID: 16282962 Review. Japanese.
-
Ten-year experience with fungal peritonitis in peritoneal dialysis patients: antifungal susceptibility patterns in a North-American center.Int J Infect Dis. 2012 Jan;16(1):e41-3. doi: 10.1016/j.ijid.2011.09.016. Epub 2011 Nov 4. Int J Infect Dis. 2012. PMID: 22056278 Review.
Cited by
-
Ameliorative role of SIRT1 in peritoneal fibrosis: an in vivo and in vitro study.Cell Biosci. 2021 Apr 27;11(1):79. doi: 10.1186/s13578-021-00591-8. Cell Biosci. 2021. PMID: 33906673 Free PMC article.
-
FAM21 is critical for TLR2/CLEC4E-mediated dendritic cell function against Candida albicans.Life Sci Alliance. 2023 Jan 30;6(4):e202201414. doi: 10.26508/lsa.202201414. Print 2023 Apr. Life Sci Alliance. 2023. PMID: 36717248 Free PMC article.
-
A case report of SMARCA2-deficient and SMARCA4-preserved lung adenocarcinoma diagnosed by pleural effusion cytology.BMC Pulm Med. 2025 Aug 26;25(1):406. doi: 10.1186/s12890-025-03891-8. BMC Pulm Med. 2025. PMID: 40859347 Free PMC article.
References
-
- Grassmann A, Gioberge S, Moeller S, Brown G. ESRD patients in 2004: global overview of patient numbers, treatment modalities and associated trends. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2005;20:2587–2593. doi: 10.1093/ndt/gfi159. - DOI - PubMed
-
- Shimaoka T, et al. Quantitative evaluation and assessment of peritoneal morphologic changes in peritoneal dialysis patients. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2010;25:3379–3385. doi: 10.1093/ndt/gfq194. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

