Thy-1 depletion and integrin β3 upregulation-mediated PI3K-Akt-mTOR pathway activation inhibits lung fibroblast autophagy in lipopolysaccharide-induced pulmonary fibrosis
- PMID: 31249375
- PMCID: PMC7102294
- DOI: 10.1038/s41374-019-0281-2
Thy-1 depletion and integrin β3 upregulation-mediated PI3K-Akt-mTOR pathway activation inhibits lung fibroblast autophagy in lipopolysaccharide-induced pulmonary fibrosis
Abstract
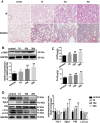
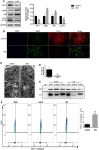
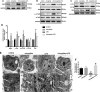
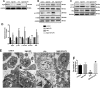
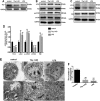
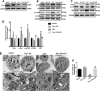
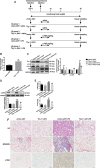
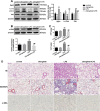
Lipopolysaccharide (LPS)-induced autophagy inhibition in lung fibroblasts is closely associated with the activation of the phosphatidylinositol 3-kinase/protein kinase B/mammalian target of rapamycin (PI3K-Akt-mTOR) pathway. However, the underlying mechanism remains unknown. In this study, we demonstrated that LPS activated the PI3K-Akt-mTOR pathway and inhibited lung fibroblast autophagy by depleting thymocyte differentiation antigen-1 (Thy-1) and upregulating integrin β3 (Itgb3). Challenge of the human lung fibroblast MRC-5 cell line with LPS resulted in significant upregulation of integrin β3, activation of the PI3K-Akt-mTOR pathway and inhibition of autophagy, which could be abolished by integrin β3 silencing by specific shRNA or treatment with the integrin β3 inhibitor cilengitide. Meanwhile, LPS could inhibit Thy-1 expression accompanied with PI3K-Akt-mTOR pathway activation and lung fibroblast autophagy inhibition; these effects could be prevented by Thy-1 overexpression. Meanwhile, Thy-1 downregulation with Thy-1 shRNA could mimic the effects of LPS, inducing the activation of PI3K-Akt-mTOR pathway and inhibiting lung fibroblast autophagy. Furthermore, protein immunoprecipitation analysis demonstrated that LPS reduced the binding of Thy-1 to integrin β3. Thy-1 downregulation, integrin β3 upregulation and autophagy inhibition were also detected in a mouse model of LPS-induced pulmonary fibrosis, which could be prohibited by intratracheal injection of Thy-1 overexpressing adeno-associated virus (AAV) or intraperitoneal injection of the integrin β3 inhibitor cilengitide. In conclusion, this study demonstrated that Thy-1 depletion and integrin β3 upregulation are involved in LPS-induced pulmonary fibrosis, and may serve as potential therapeutic targets for pulmonary fibrosis.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
-
- Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome. N Engl J Med. 2017;377:1904–5. - PubMed
-
- Armstrong L, Thickett DR, Mansell JP, Billinghurst RC, Poole AR, Millar AB. Changes in collagen turnover in early acute respiratory distress syndrome. Am J Respir Crit Care Med. 1999;160:1910–5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

