Hedgehog Pathway Activation Alters Ciliary Signaling in Primary Hypothalamic Cultures
- PMID: 31249512
- PMCID: PMC6582312
- DOI: 10.3389/fncel.2019.00266
Hedgehog Pathway Activation Alters Ciliary Signaling in Primary Hypothalamic Cultures
Abstract
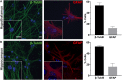
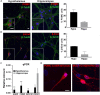
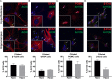
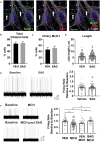
Primary cilia dysfunction has been associated with hyperphagia and obesity in both ciliopathy patients and mouse models of cilia perturbation. Neurons throughout the brain possess these solitary cellular appendages, including in the feeding centers of the hypothalamus. Several cell biology questions associated with primary neuronal cilia signaling are challenging to address in vivo. Here we utilize primary hypothalamic neuronal cultures to study ciliary signaling in relevant cell types. Importantly, these cultures contain neuronal populations critical for appetite and satiety such as pro-opiomelanocortin (POMC) and agouti related peptide (AgRP) expressing neurons and are thus useful for studying signaling involved in feeding behavior. Correspondingly, these cultured neurons also display electrophysiological activity and respond to both local and peripheral signals that act on the hypothalamus to influence feeding behaviors, such as leptin and melanin concentrating hormone (MCH). Interestingly, we found that cilia mediated hedgehog signaling, generally associated with developmental processes, can influence ciliary GPCR signaling (Mchr1) in terminally differentiated neurons. Specifically, pharmacological activation of the hedgehog-signaling pathway using the smoothened agonist, SAG, attenuated the ability of neurons to respond to ligands (MCH) of ciliary GPCRs. Understanding how the hedgehog pathway influences cilia GPCR signaling in terminally differentiated neurons could reveal the molecular mechanisms associated with clinical features of ciliopathies, such as hyperphagia-associated obesity.
Keywords: SAG; cilia; hedgehog signaling; hypothalamus; leptin; melanin concentrating hormone receptor 1; primary neuronal cultures; smoothened.
Figures

Similar articles
-
Properties of primary cilia in melanin-concentrating hormone receptor 1-bearing hippocampal neurons in vivo and in vitro.Neurochem Int. 2021 Jan;142:104902. doi: 10.1016/j.neuint.2020.104902. Epub 2020 Nov 13. Neurochem Int. 2021. PMID: 33197527
-
Modulation of primary cilia length by melanin-concentrating hormone receptor 1.Cell Signal. 2016 Jun;28(6):572-84. doi: 10.1016/j.cellsig.2016.02.018. Epub 2016 Mar 2. Cell Signal. 2016. PMID: 26946173
-
The Transition Zone Protein AHI1 Regulates Neuronal Ciliary Trafficking of MCHR1 and Its Downstream Signaling Pathway.J Neurosci. 2021 Apr 28;41(17):3932-3943. doi: 10.1523/JNEUROSCI.2993-20.2021. Epub 2021 Mar 19. J Neurosci. 2021. PMID: 33741721 Free PMC article.
-
Mechanisms of Weight Control by Primary Cilia.Mol Cells. 2022 Apr 30;45(4):169-176. doi: 10.14348/molcells.2022.2046. Mol Cells. 2022. PMID: 35387896 Free PMC article. Review.
-
[Selective signaling pathway via feeding-related ciliary GPCR, melanin-concentrating hormone receptor 1].Nihon Yakurigaku Zasshi. 2019;154(4):179-185. doi: 10.1254/fpj.154.179. Nihon Yakurigaku Zasshi. 2019. PMID: 31597896 Review. Japanese.
Cited by
-
Spinophilin-dependent regulation of GluN2B-containing NMDAR-dependent calcium influx, GluN2B surface expression, and cleaved caspase expression.Synapse. 2023 May;77(3):e22264. doi: 10.1002/syn.22264. Epub 2023 Feb 18. Synapse. 2023. PMID: 36738175 Free PMC article.
-
Signaling in the primary cilium through the lens of the Hedgehog pathway.Wiley Interdiscip Rev Dev Biol. 2020 Nov;9(6):e377. doi: 10.1002/wdev.377. Epub 2020 Feb 21. Wiley Interdiscip Rev Dev Biol. 2020. PMID: 32084300 Free PMC article. Review.
-
Cilia signaling and obesity.Semin Cell Dev Biol. 2021 Feb;110:43-50. doi: 10.1016/j.semcdb.2020.05.006. Epub 2020 May 25. Semin Cell Dev Biol. 2021. PMID: 32466971 Free PMC article. Review.
-
Artificial Intelligence Approaches to Assessing Primary Cilia.J Vis Exp. 2021 May 1;(171):10.3791/62521. doi: 10.3791/62521. J Vis Exp. 2021. PMID: 33999029 Free PMC article.
-
Epigenetic regulation of beta-endorphin synthesis in hypothalamic arcuate nucleus neurons modulates neuropathic pain in a rodent pain model.Nat Commun. 2023 Nov 9;14(1):7234. doi: 10.1038/s41467-023-43022-7. Nat Commun. 2023. PMID: 37945654 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

