Differential Effects of Purinergic Signaling in Gastric Cancer-Derived Cells Through P2Y and P2X Receptors
- PMID: 31249523
- PMCID: PMC6584115
- DOI: 10.3389/fphar.2019.00612
Differential Effects of Purinergic Signaling in Gastric Cancer-Derived Cells Through P2Y and P2X Receptors
Abstract
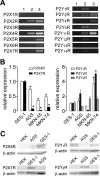
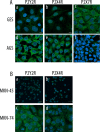
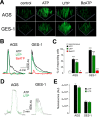
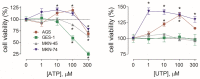
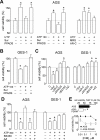
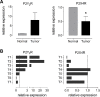
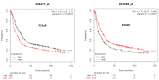
Gastric cancer (GC) is the one of the most prevalent cancers and one of the leading causes of cancer-induced deaths. Previously, we found that the expression of purinergic P2Y2 receptor (P2Y2R) is increased in GC samples as compared to adjacent healthy mucosa taken from GC-diagnosed patients. In this work, we studied in detail purinergic signaling in the gastric adenocarcinoma-derived cell lines: AGS, MKN-45, and MKN-74, and compared them to a nontumoral epithelial cell line: GES-1. In GC-derived cells, we detected the expression of several purinergic receptors, and found important differences as compared to GES-1 cells. Functional studies revealed a strong contribution of P2Y2Rs in intracellular calcium increases, elicited by adenosine-triphosphate (ATP), uridine-triphosphate (UTP), and the P2Y2R agonist MRS2768. Responses were preserved in the absence of extracellular calcium and inhibited by P2Y2R antagonists. In GES-1 cells, ATP and UTP induced similar responses and the combination of P2X and P2Y receptor antagonists was able to block them. Proliferation studies showed that ATP regulates AGS and MKN-74 cells in a biphasic manner, increasing cell proliferation at 10-100 μM, but inhibiting at 300 μM ATP. On the other hand, 1-300 μM UTP, a P2Y2R agonist, increased concentration-dependent cell proliferation. The effects of UTP and ATP were prevented by both wide-range and specific purinergic antagonists. In contrast, in GES-1 cells ATP only decreased cell proliferation in a concentration-dependent manner, and UTP had no effect. Notably, the isolated application of purinergic antagonists was sufficient to change the basal proliferation of AGS cells, indicating that nucleotides released by the cells can act as paracrine/autocrine signals. Finally, in tumor-derived biopsies, we found an increase of P2Y2R and a decrease in P2X4R expression; however, we found high variability between seven different biopsies and their respective adjacent healthy gastric mucosa. Even so, we found a correlation between the expression levels of P2Y2R and P2X4R and survival rates of GC patients. Taken together, these results demonstrate the involvement of different purinergic receptors and signaling in GC, and the pattern of expression changes in tumoral cells, and this change likely directs ATP and nucleotide signaling from antiproliferative effects in healthy tissues to proliferative effects in cancer.
Keywords: ATP; P2X receptor; P2Y receptor; gastric cancer; paracrine action; uridine triphosphate (UTP).
Figures

References
-
- Abbracchio M. P., Burnstock G., Boeynaems J. M., Barnard E. A., Boyer J. L., Kennedy C., et al. (2006). International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol. Rev. 58, 281–341. 10.1124/pr.58.3.3 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

