Estimation of the Seroconversion Duration of HIV-1 Antibodies in Individuals With Recent Infection in China
- PMID: 31249564
- PMCID: PMC6582625
- DOI: 10.3389/fmicb.2019.01322
Estimation of the Seroconversion Duration of HIV-1 Antibodies in Individuals With Recent Infection in China
Abstract
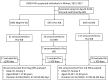
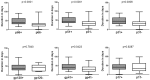
The identification of recent HIV-1 infection is clinically important for the effective treatment and prevention of transmission. However, the window period for seroconversion with respect to various HIV-1 antibodies is not well characterized. In addition, the routine HIV testing algorithms are not particularly appropriate for the identification of recent HIV-1 infection. In this study, we enrolled individuals who showed seroconversion from negative Western blot (WB) or indeterminate WB results and analyzed the window periods for appearance of HIV-1 antibodies. A total of 10,934 individuals with suspected HIV infection were tested by Wuhan CDC between 2012 and 2017; of these, 40 individuals with initial negative WB and 102 individuals with initial indeterminate WB who showed positive WB results within 100 days were included in the analysis. The mean time for seroconversion was 43.90 (95% confidence interval [CI]: 37.30-50.50) days and 42.15 (95% CI: 37.99-46.30) days, respectively. The time duration for p31 seroconversion among people with negative WB and indeterminate WB was 58.11 (95% CI, 44.30-71.92) days and 51.91 (95% CI, 44.55-59.28) days, respectively, both of which were significantly longer (p = 0.0169) than those in people without p31 seroconversion. A similar difference was observed with respect to p66 seroconversion, with a window time of 53.53 (95% CI, 43.54-63.52) days and 47.87 (95% CI, 43.16-52.57) days among people with negative WB and indeterminate WB, respectively. These data suggest that HIV-1 antibody p66, like p31, may serve as a potential serological marker for distinguishing Fiebig stage V and stage VI at day 70 post-infection.
Keywords: HIV/AIDS; Western blot bands; recent HIV-1 infection; seroconversion; viral marker.
Figures


Similar articles
-
[Study on the indeterminate results of characterization and verification of HIV antibody from Western blot test].Zhonghua Liu Xing Bing Xue Za Zhi. 2008 May;29(5):478-81. Zhonghua Liu Xing Bing Xue Za Zhi. 2008. PMID: 18956682 Chinese.
-
Frequency of human immunodeficiency virus (HIV) infection among contemporary anti-HIV-1 and anti-HIV-1/2 supplemental test-indeterminate blood donors. The Retrovirus Epidemiology Donor Study.Transfusion. 1996 Jan;36(1):37-44. doi: 10.1046/j.1537-2995.1996.36196190513.x. Transfusion. 1996. PMID: 8607151
-
The significance of western blot assays indeterminate for antibody to HIV in a cohort of homosexual/bisexual men. The Multicenter AIDS Cohort Study.J Acquir Immune Defic Syndr (1988). 1992 Oct;5(10):988-92. J Acquir Immune Defic Syndr (1988). 1992. PMID: 1453328
-
Predictive value of two commercial human immunodeficiency virus serological tests in cases with indeterminate Western blot results.J Microbiol Immunol Infect. 2006 Jun;39(3):219-24. J Microbiol Immunol Infect. 2006. PMID: 16783452
-
Prevalence of HIV Indeterminate Western Blot Tests and Follow-up of HIV Antibody Sero-Conversion in Southeastern China.Virol Sin. 2019 Aug;34(4):358-366. doi: 10.1007/s12250-019-00130-3. Epub 2019 Jun 12. Virol Sin. 2019. PMID: 31190120 Free PMC article.
Cited by
-
Breakthrough Acute HIV Infections among Pre-Exposure Prophylaxis Users with High Adherence: A Narrative Review.Viruses. 2024 Jun 12;16(6):951. doi: 10.3390/v16060951. Viruses. 2024. PMID: 38932243 Free PMC article. Review.
-
Anti-HIV-1 antibodies based confirmatory results in Wuhan, China, 2012-2018.PLoS One. 2020 Sep 11;15(9):e0238282. doi: 10.1371/journal.pone.0238282. eCollection 2020. PLoS One. 2020. PMID: 32915788 Free PMC article.
-
Using signal-to-cutoff ratios of HIV screening assay to predict HIV infection.BMC Infect Dis. 2023 Dec 13;23(1):874. doi: 10.1186/s12879-023-08891-9. BMC Infect Dis. 2023. PMID: 38093214 Free PMC article.
References
-
- Brostrom B., Granich R., Gupta S., Samb B. (2014). Reimagining HIV testing in an era of ART. AIDS Res. Hum. Retroviruses Suppl. 1:A87.
-
- Centers for Disease Control [CDC] (2014). Laboratory Testing for the Diagnosis of HIV Infection: Updated Recommendations. Atlanta, GA: Centers for Disease Control and Prevention and Association of Public Health Laboratories.
LinkOut - more resources
Full Text Sources

