Adaptive Evolution of Human-Isolated H5Nx Avian Influenza A Viruses
- PMID: 31249566
- PMCID: PMC6582624
- DOI: 10.3389/fmicb.2019.01328
Adaptive Evolution of Human-Isolated H5Nx Avian Influenza A Viruses
Abstract
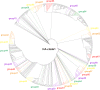
Avian influenza A viruses (AIVs) H5N1, first identified in 1996, are highly pathogenic in domestic poultry and continue to occasionally infect humans. In this study, we sought to identify genetic changes that occurred during their multiple invasions to humans. We evaluated all available H5Nx AIV genomes. Significant signals of positive selection were detected in 29 host-shift branches. 126 parallel evolution sites were detected on these branches, including 17 well-known sites (such as T271A, A274T, T339M, Q591K, E627K, and D701N in PB2; A134V, D154N, S223N, and R497K in HA) that play roles in allowing AIVs to cross species barriers. Our study suggests that during human infections, H5Nx viruses have experienced adaptive evolution (positive selection and convergent evolution) that allowed them to adapt to their new host environments. Analyses of adaptive evolution should be useful in identifying candidate sites that play roles in human infections, which can be tested by functional experiments.
Keywords: Avian influenza A virus; H5Nx; convergent evolution; host shift; human infection.
Figures
References
LinkOut - more resources
Full Text Sources

