Linked within-host and between-host models and data for infectious diseases: a systematic review
- PMID: 31249734
- PMCID: PMC6589080
- DOI: 10.7717/peerj.7057
Linked within-host and between-host models and data for infectious diseases: a systematic review
Abstract
The observed dynamics of infectious diseases are driven by processes across multiple scales. Here we focus on two: within-host, that is, how an infection progresses inside a single individual (for instance viral and immune dynamics), and between-host, that is, how the infection is transmitted between multiple individuals of a host population. The dynamics of each of these may be influenced by the other, particularly across evolutionary time. Thus understanding each of these scales, and the links between them, is necessary for a holistic understanding of the spread of infectious diseases. One approach to combining these scales is through mathematical modeling. We conducted a systematic review of the published literature on multi-scale mathematical models of disease transmission (as defined by combining within-host and between-host scales) to determine the extent to which mathematical models are being used to understand across-scale transmission, and the extent to which these models are being confronted with data. Following the PRISMA guidelines for systematic reviews, we identified 24 of 197 qualifying papers across 30 years that include both linked models at the within and between host scales and that used data to parameterize/calibrate models. We find that the approach that incorporates both modeling with data is under-utilized, if increasing. This highlights the need for better communication and collaboration between modelers and empiricists to build well-calibrated models that both improve understanding and may be used for prediction.
Keywords: Between-host; Data-model integration; Infectious disease models; Linking mechanism; Mulit-scale modeling; Pathogen transmission; SIR models; Within-host.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

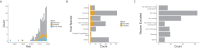





References
-
- Anderson RM, May RM. Infectious diseases of humans: dynamics and control. New York: Oxford University Press; 1992.
-
- Chaves LF, Kaneko A, Pascual M. Random, top-down, or bottom-up coexistence of parasites: malaria population dynamics in multi-parasitic settings. Ecology. 2009;90(9):2414–2425. - PubMed
LinkOut - more resources
Full Text Sources

