Efficacy of Dexamethasone for Acute Primary Immune Thrombocytopenia Compared to Prednisolone: A Systematic Review and Meta-analysis
- PMID: 31249913
- PMCID: PMC6524842
- DOI: 10.1055/s-0037-1604168
Efficacy of Dexamethasone for Acute Primary Immune Thrombocytopenia Compared to Prednisolone: A Systematic Review and Meta-analysis
Abstract
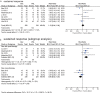
Corticosteroids have been established as first-line therapy in acute primary immune thrombocytopenia (ITP), and the clinical guidelines recommend either dexamethasone (Dex) or prednisolone (PSL). The types and dosages of corticosteroids, however, have not yet been determined, because previous randomized control trials (RCTs) comparing Dex and PSL showed controversial results in terms of efficacy. To understand and interpret all available evidence, we conducted a systematic review and meta-analysis of RCTs. The main outcome measure was the incidence of sustained response (SR; platelet count >30 × 10 9 /L for 6 months without concomitant treatments after the completion of the final therapies). Eight RCTs (totaling 704 patients) were included in this study. The incidence of SR showed no significant difference, while it was significantly higher in the Dex arm when used with posttherapy (more than one course of Dex or tapering corticosteroids added; risk ratio [RR], 1.82; 95% confidence interval [CI], 1.38-2.41; p < 0.01). A single course of Dex showed no significant difference. The overall response (platelet >30 × 10 9 /L) at day 28 was significantly improved in the Dex arm (RR, 1.11; 95% CI, 1.01-1.22; p = 0.03) and Dex with posttherapy suppressed long-term relapse (RR of nonevent, 1.32; 95% CI, 1.10-1.59; p < 0.01). There were significantly fewer adverse events in the Dex arm (RR, 0.45; 95% CI, 0.37-0.55; p < 0.01). Use of Dex with posttherapy instead of PSL may be more beneficial as the initial therapy. Studies comparing Dex with other new strategies are essential to determine the most suitable therapeutic regimens for acute ITP.
Keywords: dexamethasone; meta-analysis; prednisolone; thrombocytopenia.
Conflict of interest statement
Figures

References
-
- Stasi R, Evangelista M L, Stipa E, Buccisano F, Venditti A, Amadori S. Idiopathic thrombocytopenic purpura: current concepts in pathophysiology and management. Thromb Haemost. 2008;99(01):4–13. - PubMed
-
- Cohen Y C, Djulbegovic B, Shamai-Lubovitz O, Mozes B. The bleeding risk and natural history of idiopathic thrombocytopenic purpura in patients with persistent low platelet counts. Arch Intern Med. 2000;160(11):1630–1638. - PubMed
-
- Psaila B, Bussel J B. Immune thrombocytopenic purpura. Hematol Oncol Clin North Am. 2007;21(04):743–759. - PubMed
-
- Provan D, Stasi R, Newland A C et al.International consensus report on the investigation and management of primary immune thrombocytopenia. Blood. 2010;115(02):168–186. - PubMed
-
- Neunert C, Lim W, Crowther M, Cohen A, Solberg L, Jr, Crowther M A; American Society of Hematology.The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia Blood 2011117164190–4207. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

