BCR-ABL Tyrosine Kinase Inhibitors: Which Mechanism(s) May Explain the Risk of Thrombosis?
- PMID: 31249931
- PMCID: PMC6524858
- DOI: 10.1055/s-0038-1624566
BCR-ABL Tyrosine Kinase Inhibitors: Which Mechanism(s) May Explain the Risk of Thrombosis?
Abstract
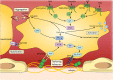
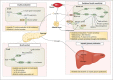
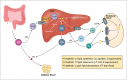
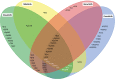
Imatinib, the first-in-class BCR-ABL tyrosine kinase inhibitor (TKI), had been a revolution for the treatment of chronic myeloid leukemia (CML) and had greatly enhanced patient survival. Second- (dasatinib, nilotinib, and bosutinib) and third-generation (ponatinib) TKIs have been developed to be effective against BCR-ABL mutations making imatinib less effective. However, these treatments have been associated with arterial occlusive events. This review gathers clinical data and experiments about the pathophysiology of these arterial occlusive events with BCR-ABL TKIs. Imatinib is associated with very low rates of thrombosis, suggesting a potentially protecting cardiovascular effect of this treatment in patients with BCR-ABL CML. This protective effect might be mediated by decreased platelet secretion and activation, decreased leukocyte recruitment, and anti-inflammatory or antifibrotic effects. Clinical data have guided mechanistic studies toward alteration of platelet functions and atherosclerosis development, which might be secondary to metabolism impairment. Dasatinib, nilotinib, and ponatinib affect endothelial cells and might induce atherogenesis through increased vascular permeability. Nilotinib also impairs platelet functions and induces hyperglycemia and dyslipidemia that might contribute to atherosclerosis development. Description of the pathophysiology of arterial thrombotic events is necessary to implement risk minimization strategies.
Keywords: BCR-ABL; arterial thrombotic events; chronic myeloid leukemia; tyrosine kinase inhibitors.
Conflict of interest statement
F.M. reports personal fees from Boehringer Ingelheim, Bayer Healthcare, and Bristol-Myers Squibb-Pfizer outside the submitted work.
C.G. reports personal fees from Novartis, Celgene, and Amgen outside the submitted work.
The other authors have no conflicts of interest to disclose.
Figures
References
-
- Gorre M E, Mohammed M, Ellwood Ket al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification Science 2001293(5531):876–880. - PubMed
-
- Bixby D, Talpaz M. Mechanisms of resistance to tyrosine kinase inhibitors in chronic myeloid leukemia and recent therapeutic strategies to overcome resistance. Hematology (Am Soc Hematol Educ Program) 2009;2009(01):461–476. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

