Patient Management Strategies and Long-Term Outcomes in Isolated Distal Deep-Vein Thrombosis versus Proximal Deep-Vein Thrombosis: Findings from XALIA
- PMID: 31249987
- PMCID: PMC6524913
- DOI: 10.1055/s-0039-1683968
Patient Management Strategies and Long-Term Outcomes in Isolated Distal Deep-Vein Thrombosis versus Proximal Deep-Vein Thrombosis: Findings from XALIA
Abstract
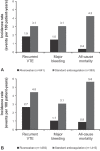
Background Overall, 30 to 50% of lower-limb deep-vein thrombosis (DVT) cases are isolated distal DVT (IDDVT). The recurrent venous thromboembolism (VTE) risk is unclear, leaving uncertainty over optimal IDDVT treatment. We present data on patients with IDDVT and proximal DVT (PDVT) from the prospective, noninterventional XALIA study of rivaroxaban for acute and extended VTE treatment. Methods Patients aged ≥18 years scheduled to receive ≥3 months' anticoagulation with rivaroxaban or standard anticoagulation were eligible, with follow-up for ≥12 months. We describe baseline characteristics, management strategies, and incidence proportions of VTE recurrence, major bleeding, and all-cause mortality in patients with IDDVT or PDVT, with or without distal vein involvement. Findings Overall, 1,004 patients with IDDVT and 3,098 with PDVT were enrolled; 641 (63.8%) and 1,683 (54.3%) received rivaroxaban, respectively. Patients with IDDVT were younger and had lower incidences of renal impairment, cancer, and unprovoked VTE than those with PDVT. On-treatment recurrence incidences for IDDVT versus PDVT were 1.0 versus 2.4% (adjusted hazard ratio [HR]: 0.56; 95% confidence interval [CI]: 0.29-1.08), and incidences posttreatment cessation were 1.1 versus 2.1% (adjusted HR: 0.65; 95% CI: 0.32-1.35), respectively. On-treatment major bleeding incidences were 0.9 versus 1.4% and mortality was 0.8 versus 2.2%, respectively. Median treatment duration in patients with IDDVT was shorter than in those with PDVT (102 vs. 192 days, respectively). Interpretation Patients with IDDVT had fewer comorbidities and were more frequently treated with rivaroxaban than those with PDVT. On-treatment and posttreatment recurrences were less frequent in patients with IDDVT. Trial registration number: NCT01619007.
Keywords: deep-vein thrombosis; rivaroxaban; routine clinical practice; venous thromboembolism.
Conflict of interest statement
Figures
References
-
- Palareti G, Schellong S. Isolated distal deep vein thrombosis: what we know and what we are doing. J Thromb Haemost. 2012;10(01):11–19. - PubMed
-
- Barco S, Corti M, Trinchero A et al. Survival and recurrent venous thromboembolism in patients with first proximal or isolated distal deep vein thrombosis and no pulmonary embolism. J Thromb Haemost. 2017;15(07):1436–1442. - PubMed
-
- Franco L, Giustozzi M, Agnelli G, Becattini C. Anticoagulation in patients with isolated distal deep vein thrombosis: a meta-analysis. J Thromb Haemost. 2017;15(06):1142–1154. - PubMed
-
- Donadini M P, Dentali F, Pegoraro S et al. Long-term recurrence of venous thromboembolism after short-term treatment of symptomatic isolated distal deep vein thrombosis: a cohort study. Vasc Med. 2017;22(06):518–524. - PubMed
-
- Galanaud J P, Sevestre M A, Genty C et al. Incidence and predictors of venous thromboembolism recurrence after a first isolated distal deep vein thrombosis. J Thromb Haemost. 2014;12(04):436–443. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

