Antiphosphatidylserine Immunoglobulin M and Immunoglobulin G Antibodies Are Higher in Vivax Than Falciparum Malaria, and Associated With Early Anemia in Both Species
- PMID: 31250022
- PMCID: PMC8453602
- DOI: 10.1093/infdis/jiz334
Antiphosphatidylserine Immunoglobulin M and Immunoglobulin G Antibodies Are Higher in Vivax Than Falciparum Malaria, and Associated With Early Anemia in Both Species
Abstract
Background: Anemia is a major complication of vivax malaria. Antiphosphatidylserine (PS) antibodies generated during falciparum malaria mediate phagocytosis of uninfected red blood cells that expose PS and have been linked to late malarial anemia. However, their role in anemia from non-falciparum Plasmodium species is not known, nor their role in early anemia from falciparum malaria.
Methods: We measured PS immunoglobulin G (IgG) and immunoglobulin M (IgM) antibodies in Malaysian patients with vivax, falciparum, knowlesi, and malariae malaria, and in healthy controls, and correlated antibody titres with hemoglobin. PS antibodies were also measured in volunteers experimentally infected with Plasmodium vivax and Plasmodium falciparum.
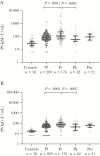
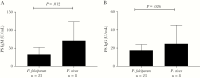
Results: PS IgM and IgG antibodies were elevated in patients with vivax, falciparum, knowlesi, and malariae malaria (P < .0001 for all comparisons with controls) and were highest in vivax malaria. In vivax and falciparum malaria, PS IgM and IgG on admission correlated inversely with admission and nadir hemoglobin, controlling for parasitemia and fever duration. PS IgM and IgG were also increased in volunteers infected with blood-stage P. vivax and P. falciparum, and were higher in P. vivax infection.
Conclusions: PS antibodies are higher in vivax than falciparum malaria, correlate inversely with hemoglobin, and may contribute to the early loss of uninfected red blood cells found in malarial anemia from both species.
Keywords: Plasmodium falciparum; Plasmodium knowlesi; Plasmodium malariae; Plasmodium vivax; anemia; malaria; phosphatidylserine antibodies.
© The Author(s) 2019. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures

References
-
- Jakeman GN, Saul A, Hogarth WL, Collins WE. Anaemia of acute malaria infections in non-immune patients primarily results from destruction of uninfected erythrocytes. Parasitology 1999; 119 (Pt 2):127–33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

