Public Health Impact and Cost-Effectiveness of Non-live Adjuvanted Recombinant Zoster Vaccine in Canadian Adults
- PMID: 31250218
- PMCID: PMC6748891
- DOI: 10.1007/s40258-019-00491-6
Public Health Impact and Cost-Effectiveness of Non-live Adjuvanted Recombinant Zoster Vaccine in Canadian Adults
Abstract
Objectives: In Canada, incidences of herpes zoster (HZ) and postherpetic neuralgia (PHN) are increasing, posing a significant burden on the healthcare system. This study aimed to determine the public health impact and cost effectiveness of an adjuvanted recombinant zoster vaccine (RZV) compared to no vaccination and to the live attenuated vaccine (ZVL) in Canadians aged 60 years and older.
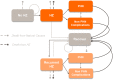
Methods: A multi-cohort Markov model has been adapted to the Canadian context using recent demographic and epidemiologic data. Simulations consisted of age-cohorts annually transitioning between health states. Health outcomes and costs were discounted at 1.5% per year. The perspective of the Canadian healthcare payer was adopted. A coverage of 80% for the first RZV and ZVL dose and a compliance of 75% for the second RZV dose were assumed.
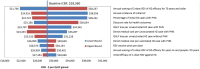
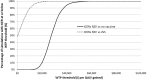
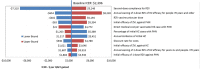
Results: RZV was estimated to be cost effective compared with no vaccination with an incremental cost-effectiveness ratio (ICER) of $28,360 (Canadian dollars) per quality-adjusted life-year (QALY) in persons aged ≥ 60 years, avoiding 554,504 HZ and 166,196 PHN cases. Compared with ZVL, RZV accrued more QALYs through the remaining lifetime and an increase in costs of approximately $50 million resulting in an average ICER of $2396. Results were robust under deterministic and probabilistic sensitivity analyses. HZ incidence rate and persistence of vaccine efficacy had the largest impact on cost effectiveness.
Conclusions: The cost-utility analysis suggested that RZV would be cost effective in the Canadian population compared with no vaccination and vaccination with ZVL at a willingness-to-pay threshold of $50,000.
Plain language summary
More than 95% of adults aged 50 are infected with varicella-zoster virus and are at risk of developing herpes zoster, also known as shingles. This risk is higher in older people and in people with a reduced immune system. Shingles causes a painful rash and may trigger persistent pain and other complications that greatly reduce quality of life. In Canada, Zostavax is the only existing approved vaccine against shingles. It has been offered in a publicly funded program in Ontario to those aged 65–70 years since September 2016. Shingrix, is a new shingles vaccine that has recently been approved by Health Canada for adults aged ≥ 50 years. The present model suggests that Shingrix confers higher protection against shingles compared to Zostavax, with a greater reduction in shingles episodes. The increase in vaccination costs would be partially offset by reduced healthcare visit and medication expenses. For these reasons, provincial health plans may consider offering Shingrix to people aged ≥ 50 years.
Conflict of interest statement
AMG, DVO, RW, LV and DC are employees of the GSK group of companies and DC holds shares in the GSK group of companies. MS and MG are employees of Evidera, a consulting firm that received fees from the GSK group of companies to conduct of these analyses. During the conduct of this study, HJ was also an employee of Evidera.
Figures
Similar articles
-
Public health impact of herpes zoster vaccination on older adults in Singapore: a modeling study.Hum Vaccin Immunother. 2024 Dec 31;20(1):2348839. doi: 10.1080/21645515.2024.2348839. Epub 2024 May 28. Hum Vaccin Immunother. 2024. PMID: 38804600 Free PMC article.
-
Public health impact of herpes zoster vaccination on older adults in Hong Kong.Hum Vaccin Immunother. 2023 Dec 31;19(1):2176065. doi: 10.1080/21645515.2023.2176065. Epub 2023 Feb 28. Hum Vaccin Immunother. 2023. PMID: 36854447 Free PMC article.
-
Cost-effectiveness of an Adjuvanted Recombinant Zoster Vaccine in older adults in the United States.Vaccine. 2018 Aug 9;36(33):5037-5045. doi: 10.1016/j.vaccine.2018.07.005. Epub 2018 Jul 14. Vaccine. 2018. PMID: 30017145
-
Development of adjuvanted recombinant zoster vaccine and its implications for shingles prevention.Expert Rev Vaccines. 2018 Jul;17(7):619-634. doi: 10.1080/14760584.2018.1495565. Epub 2018 Jul 20. Expert Rev Vaccines. 2018. PMID: 30028651 Review.
-
Cost-effectiveness of the recombinant zoster vaccine (RZV) against herpes zoster: An updated critical review.Hum Vaccin Immunother. 2023 Dec 31;19(1):2168952. doi: 10.1080/21645515.2023.2168952. Epub 2023 Mar 14. Hum Vaccin Immunother. 2023. PMID: 36916240 Free PMC article. Review.
Cited by
-
Global herpes zoster incidence, burden of disease, and vaccine availability: a narrative review.Ther Adv Vaccines Immunother. 2022 Mar 21;10:25151355221084535. doi: 10.1177/25151355221084535. eCollection 2022. Ther Adv Vaccines Immunother. 2022. PMID: 35340552 Free PMC article. Review.
-
Updated Public Health Impact and Cost Effectiveness of Recombinant Zoster Vaccine in Canadian Adults Aged 50 Years and Older.Pharmacoecon Open. 2024 May;8(3):481-492. doi: 10.1007/s41669-024-00483-w. Epub 2024 Apr 11. Pharmacoecon Open. 2024. PMID: 38605257 Free PMC article.
-
How large could the public health impact of introducing recombinant zoster vaccination for people aged ≥50 years in five Latin American countries be?Hum Vaccin Immunother. 2023 Dec 31;19(1):2164144. doi: 10.1080/21645515.2022.2164144. Epub 2023 Feb 23. Hum Vaccin Immunother. 2023. PMID: 36821856 Free PMC article.
-
Cost-Effectiveness Analysis of Herpes Zoster Vaccination in 50- to 85-Year-Old Immunocompetent Belgian Cohorts: A Comparison between No Vaccination, the Adjuvanted Subunit Vaccine, and Live-Attenuated Vaccine.Pharmacoeconomics. 2022 Apr;40(4):461-476. doi: 10.1007/s40273-021-01099-2. Epub 2022 Jan 30. Pharmacoeconomics. 2022. PMID: 35094374
-
Public health impact of herpes zoster vaccination on older adults in Singapore: a modeling study.Hum Vaccin Immunother. 2024 Dec 31;20(1):2348839. doi: 10.1080/21645515.2024.2348839. Epub 2024 May 28. Hum Vaccin Immunother. 2024. PMID: 38804600 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

