Gold(I) Cationization Promotes Ring Opening in Lysine-Containing Cyclic Peptides
- PMID: 31250319
- PMCID: PMC6812625
- DOI: 10.1007/s13361-019-02247-x
Gold(I) Cationization Promotes Ring Opening in Lysine-Containing Cyclic Peptides
Abstract
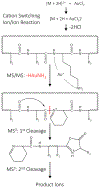
A strategy to sequence lysine-containing cyclic peptides by MSn is presented. Doubly protonated cyclic peptides ions are transformed into gold (I) cationized peptide ions via cation switching ion/ion reaction. Gold(I) cationization facilitates the oxidation of neutral lysine residues in the gas phase, weakening the adjacent amide bond. Upon activation, facile cleavage N-terminal to the oxidized lysine residue provides a site-specific ring opening pathway that converts cyclic peptides into acyclic analogs. The ensuing ion contains a cyclic imine as the new N-terminus and an oxazolone, or structural equivalent, as the new C-terminus. Product ions are formed from subsequent fragmentation events of the linearized peptide ion. Such an approach simplifies MS/MS data interpretation as a series of fragment ions with common N- and C-termini are generated. Results are presented for two cyclic peptides, sunflower trypsin inhibitor and the model cyclic peptide, β-Loop. The power of this strategy lies in the ability to generate the oxidized peptide, which is easily identified via the loss of HAuNH3 from [M + Au]+. While some competitive processes are observed, the site of ring opening can be pinpointed to the lysine residue upon MS4 enabling the unambiguous sequencing of cyclic peptides.
Keywords: Cyclic peptides; Gold cationization; Ion/ion reactions; Sunflower trypsin inhibitor.
Figures
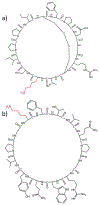
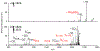
 ) indicates the species subjected to CID.
) indicates the species subjected to CID.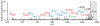
 ) indicates the species subjected to CID. Product ions corresponding to opening at lysine are highlighted in red. Fragment ions corresponding to opening at Dha are highlighted in blue and green.
) indicates the species subjected to CID. Product ions corresponding to opening at lysine are highlighted in red. Fragment ions corresponding to opening at Dha are highlighted in blue and green.
 ) indicates the species subjected to CID.
) indicates the species subjected to CID.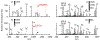
 ) indicates the species subjected to CID. Lysine residue loss (i.e. 147 Da lower in mass) is represented with a triangle superscript (Δ).
) indicates the species subjected to CID. Lysine residue loss (i.e. 147 Da lower in mass) is represented with a triangle superscript (Δ).
References
-
- Craik DJ, Daly NL, Bond T, Waine C: Plant cyclotides: A unique family of cyclic and knotted proteins that defines the cyclic cystine knot structural motif. J. Mol. Biol 294, 1327–1336 (1999) - PubMed
-
- Craik DJ: Discovery and applications of the plant cyclotides. Toxicon 56, 1092–1102 (2010) - PubMed
-
- Colgrave ML, Craik DJ: Thermal, Chemical, and Enzymatic Stability of the Cyclotide Kalata B1: The Importance of the Cyclic Cystine Knot. Biochemistry 43, 5965–5975 (2004) - PubMed
-
- Craik DJ, Conibear AC: The Chemistry of Cyclotides. J. Org. Chem 76, 4805–4817 (2011) - PubMed
-
- Pränting M, Lööv C, Burman R, Göransson U, Andersson DI: The cyclotide cycloviolacin O2 from Viola odorata has potent bactericidal activity against Gram-negative bacteria. J. Antimicrob. Chemother 65, 1964–1971 (2010) - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

