Diagnostic Yield of Next Generation Sequencing in Genetically Undiagnosed Patients with Primary Immunodeficiencies: a Systematic Review
- PMID: 31250335
- PMCID: PMC6697711
- DOI: 10.1007/s10875-019-00656-x
Diagnostic Yield of Next Generation Sequencing in Genetically Undiagnosed Patients with Primary Immunodeficiencies: a Systematic Review
Abstract
Background: As the application of next generation sequencing (NGS) is moving to earlier stages in the diagnostic pipeline for primary immunodeficiencies (PIDs), re-evaluation of its effectiveness is required. The aim of this study is to systematically review the diagnostic yield of NGS in PIDs.
Methods: PubMed and Embase databases were searched for relevant studies. Studies were eligible when describing the use of NGS in patients that had previously been diagnosed with PID on clinical and/or laboratory findings. Relevant data on study characteristics, technological performance and diagnostic yield were extracted.
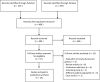
Results: Fourteen studies were eligible for data extraction. Six studies described patient populations from specific PID subcategories. The remaining studies included patients with unsorted PIDs. The studies were based on populations from Italy, Iran, Turkey, Thailand, the Netherlands, Norway, Saudi Arabia, Sweden, the UK, and the USA. Eight studies used an array-based targeted gene panel, four used WES in combination with a PID filter, and two used both techniques. The mean reported reading depth ranged from 98 to 1337 times. Five studies described the sensitivity of the applied techniques, ranging from 83 to 100%, whereas specificity ranged from 45 to 99.9%. The percentage of patients who were genetically diagnosed ranged from 15 to 79%. Several studies described clinical implications of the genetic findings.
Discussion: NGS has the ability to contribute significantly to the identification of molecular mechanisms in PID patients. The diagnostic yield highly depends on population and on the technical circumstances under which NGS is employed. Further research is needed to determine the exact diagnostic yield and clinical implications of NGS in patients with PID.
Keywords: Primary immunodeficiency (PID); clinical performance; diagnostic yield; next generation sequencing (NGS); technological performance; whole exome sequencing (WES); whole genome sequencing (WGS).
Conflict of interest statement
J.M. van Montfrans served on an advisory board for Shire.
References
-
- Shillitoe B, Bangs C, Guzman D, Gennery AR, Longhurst HJ, Slatter M, Edgar DM, Thomas M, Worth A, Huissoon A, Arkwright PD, Jolles S, Bourne H, Alachkar H, Savic S, Kumararatne DS, Patel S, Baxendale H, Noorani S, Yong PFK, Waruiru C, Pavaladurai V, Kelleher P, Herriot R, Bernatonienne J, Bhole M, Steele C, Hayman G, Richter A, Gompels M, Chopra C, Garcez T, Buckland M. The United Kingdom primary immune deficiency (UKPID) registry 2012 to 2017. Clin Exp Immunol. 2018;192:284–291. doi: 10.1111/cei.13125. - DOI - PMC - PubMed
-
- Heimall JR, Hagin D, Hajjar J, Henrickson SE, Hernandez-Trujillo HS, Tan Y, Kobrynski L, Paris K, Torgerson TR, Verbsky JW, Wasserman RL, Hsieh EWY, Blessing JJ, Chou JS, Lawrence MG, Marsh RA, Rosenzweig SD, Orange JS, Abraham RS. Use of genetic testing for primary immunodeficiency patients. J Clin Immunol. 2018;38:320–329. doi: 10.1007/s10875-018-0489-8. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials