Campylobacter fetus is Internalized by Bovine Endometrial Epithelial Cells
- PMID: 31250592
- PMCID: PMC7256759
- DOI: 10.33073/pjm-2019-022
Campylobacter fetus is Internalized by Bovine Endometrial Epithelial Cells
Abstract
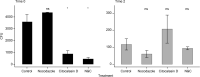
Campylobacter fetus is an important venereal pathogen of cattle that causes infertility and abortions. It is transmitted during mating, and it travels from the vagina to the uterus; therefore, an important cell type that interacts with C. fetus are endometrial epithelial cells. Several virulence factors have been identified in the genome of C. fetus, such as adhesins, secretion systems, and antiphagocytic layers, but their expression is unknown. The ability of C. fetus to invade human epithelial cells has been demonstrated, but the ability of this microorganism to infect bovine endometrial epithelial cells has not been demonstrated. Bovine endometrial epithelial cells were isolated and challenged with C. fetus. The presence of C. fetus inside the endometrial epithelial cells was confirmed by the confocal immunofluorescence. C. fetus was not internalized when actin polymerization was disturbed, suggesting cytoskeleton participation in an internalization mechanism. To evaluate the intracellular survival of C. fetus, a gentamicin protection assay was performed. Although C. fetus was able to invade epithelial cells, the results showed that it did not have the capacity to survive in the intracellular environment. This study reports for the first time, the ability of C. fetus to invade bovine endometrial epithelial cells, and actin participation in this phenomenon.
Campylobacter fetus is an important venereal pathogen of cattle that causes infertility and abortions. It is transmitted during mating, and it travels from the vagina to the uterus; therefore, an important cell type that interacts with C. fetus are endometrial epithelial cells. Several virulence factors have been identified in the genome of C. fetus, such as adhesins, secretion systems, and antiphagocytic layers, but their expression is unknown. The ability of C. fetus to invade human epithelial cells has been demonstrated, but the ability of this microorganism to infect bovine endometrial epithelial cells has not been demonstrated. Bovine endometrial epithelial cells were isolated and challenged with C. fetus. The presence of C. fetus inside the endometrial epithelial cells was confirmed by the confocal immunofluorescence. C. fetus was not internalized when actin polymerization was disturbed, suggesting cytoskeleton participation in an internalization mechanism. To evaluate the intracellular survival of C. fetus, a gentamicin protection assay was performed. Although C. fetus was able to invade epithelial cells, the results showed that it did not have the capacity to survive in the intracellular environment. This study reports for the first time, the ability of C. fetus to invade bovine endometrial epithelial cells, and actin participation in this phenomenon.
Conflict of interest statement
The authors do not report any financial or personal connections with other persons or organizations, which might negatively affect the contents of this publication and/or claim authorship rights to this publication.
Figures



Similar articles
-
Campylobacter fetus Induced Proinflammatory Response in Bovine Endometrial Epithelial Cells.Pol J Microbiol. 2021 Mar;70(1):99-106. doi: 10.33073/pjm-2021-009. Epub 2021 Mar 19. Pol J Microbiol. 2021. PMID: 33815531 Free PMC article.
-
Characterization of Campylobacter fetus adherence, invasiveness, and ultrastructural damage on bovine oviductal cells.Microb Pathog. 2025 May;202:107429. doi: 10.1016/j.micpath.2025.107429. Epub 2025 Feb 26. Microb Pathog. 2025. PMID: 40021032
-
Evaluation of intracellular survival of Campylobacter fetus subsp. fetus in bovine endometrial cells by qPCR.Iran J Vet Res. 2021 Spring;22(2):94-99. doi: 10.22099/ijvr.2021.38693.5632. Iran J Vet Res. 2021. PMID: 34306105 Free PMC article.
-
A review of sexually transmitted bovine trichomoniasis and campylobacteriosis affecting cattle reproductive health.Theriogenology. 2016 Mar 15;85(5):781-791. doi: 10.1016/j.theriogenology.2015.10.037. Epub 2015 Nov 5. Theriogenology. 2016. PMID: 26679515 Review.
-
New molecular microbiology approaches in the study of Campylobacter fetus.Microb Biotechnol. 2011 Jan;4(1):8-19. doi: 10.1111/j.1751-7915.2010.00173.x. Microb Biotechnol. 2011. PMID: 21255368 Free PMC article. Review.
Cited by
-
Campylobacter fetus Induced Proinflammatory Response in Bovine Endometrial Epithelial Cells.Pol J Microbiol. 2021 Mar;70(1):99-106. doi: 10.33073/pjm-2021-009. Epub 2021 Mar 19. Pol J Microbiol. 2021. PMID: 33815531 Free PMC article.
-
An in vitro comparison of antimicrobial efficacy and cytotoxicity between povidone-iodine and chlorhexidine for treating clinical endometritis in dairy cows.PLoS One. 2022 Jul 8;17(7):e0271274. doi: 10.1371/journal.pone.0271274. eCollection 2022. PLoS One. 2022. PMID: 35802692 Free PMC article.
References
-
- Ali A, Soares SC, Santos AR, Guimarães LC, Barbosa E, Almeida SS, Abreu VAC, Carneiro AR, Ramos RTJ, Bakhtiar SM, et al. .. Campylobacter fetus subspecies: comparative genomics and prediction of potential virulence targets. Gene. 2012. Oct;508(2): 145–156. doi:10.1016/j.gene.2012.07.070 Medline - DOI - PubMed
-
- Chiapparrone ML, Soto P, Catena M.. Characterization of the Campylobacter fetus subsp. venerealis adhesion to bovine sperm cells. Int. J. Morphol. 2016;34(4):1419–1423. doi:10.4067/S0717-95022016000400040 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
