Branched-chain amino acid and branched-chain ketoacid ingestion increases muscle protein synthesis rates in vivo in older adults: a double-blind, randomized trial
- PMID: 31250889
- PMCID: PMC6766442
- DOI: 10.1093/ajcn/nqz120
Branched-chain amino acid and branched-chain ketoacid ingestion increases muscle protein synthesis rates in vivo in older adults: a double-blind, randomized trial
Abstract
Background: Protein ingestion increases muscle protein synthesis rates. However, limited data are currently available on the effects of branched-chain amino acid (BCAA) and branched-chain ketoacid (BCKA) ingestion on postprandial muscle protein synthesis rates.
Objective: The aim of this study was to compare the impact of ingesting 6 g BCAA, 6 g BCKA, and 30 g milk protein (MILK) on the postprandial rise in circulating amino acid concentrations and subsequent myofibrillar protein synthesis rates in older males.
Methods: In a parallel design, 45 older males (age: 71 ± 1 y; BMI: 25.4 ± 0.8 kg/m2) were randomly assigned to ingest a drink containing 6 g BCAA, 6 g BCKA, or 30 g MILK. Basal and postprandial myofibrillar protein synthesis rates were assessed by primed continuous l-[ring-13C6]phenylalanine infusions with the collection of blood samples and muscle biopsies.
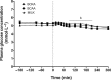
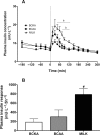
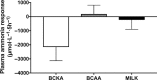
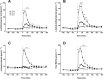
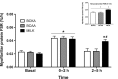
Results: Plasma BCAA concentrations increased following test drink ingestion in all groups, with greater increases in the BCAA and MILK groups compared with the BCKA group (P < 0.05). Plasma BCKA concentrations increased following test drink ingestion in all groups, with greater increases in the BCKA group compared with the BCAA and MILK groups (P < 0.05). Ingestion of MILK, BCAA, and BCKA significantly increased early myofibrillar protein synthesis rates (0-2 h) above basal rates (from 0.020 ± 0.002%/h to 0.042 ± 0.004%/h, 0.022 ± 0.002%/h to 0.044 ± 0.004%/h, and 0.023 ± 0.003%/h to 0.044 ± 0.004%/h, respectively; P < 0.001), with no differences between groups (P > 0.05). Myofibrillar protein synthesis rates during the late postprandial phase (2-5 h) remained elevated in the MILK group (0.039 ± 0.004%/h; P < 0.001), but returned to baseline values following BCAA and BCKA ingestion (0.024 ± 0.005%/h and 0.024 ± 0.005%/h, respectively; P > 0.05).
Conclusions: Ingestion of 6 g BCAA, 6 g BCKA, and 30 g MILK increases myofibrillar protein synthesis rates during the early postprandial phase (0-2 h) in vivo in healthy older males. The postprandial increase following the ingestion of 6 g BCAA and BCKA is short-lived, with higher myofibrillar protein synthesis rates only being maintained following the ingestion of an equivalent amount of intact milk protein. This trial was registered at Nederlands Trial Register (www.trialregister.nl) as NTR6047.
Keywords: aging; anabolism; chronic kidney disease; dietary protein; leucine; milk; sarcopenia; α-ketoisocaproic acid.
Copyright © American Society for Nutrition 2019.
Figures



Comment in
-
Branched-chain ketoacid ingestion: an alternative to efficiently increase skeletal muscle protein synthesis.Am J Clin Nutr. 2019 Oct 1;110(4):799-800. doi: 10.1093/ajcn/nqz190. Am J Clin Nutr. 2019. PMID: 31504096 No abstract available.
References
-
- Rennie MJ, Bohe J, Wolfe RR. Latency, duration and dose response relationships of amino acid effects on human muscle protein synthesis. J Nutr. 2002;132:3225S–7S. - PubMed
-
- Wolfe RR. Regulation of muscle protein by amino acids. J Nutr. 2002;132:3219S–24S. - PubMed
-
- Bennet WM, Connacher AA, Scrimgeour CM, Smith K, Rennie MJ. Increase in anterior tibialis muscle protein synthesis in healthy man during mixed amino acid infusion: Studies of incorporation of [1-13C]leucine. Clin Sci (Lond). 1989;76:447–54. - PubMed
-
- Atherton PJ, Smith K, Etheridge T, Rankin D, Rennie MJ. Distinct anabolic signalling responses to amino acids in C2C12 skeletal muscle cells. Amino Acids. 2010;38:1533–9. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

