Circulating tumor DNA as a potential marker of adjuvant chemotherapy benefit following surgery for localized pancreatic cancer
- PMID: 31250894
- PMCID: PMC6771221
- DOI: 10.1093/annonc/mdz200
Circulating tumor DNA as a potential marker of adjuvant chemotherapy benefit following surgery for localized pancreatic cancer
Abstract
Background: In early-stage pancreatic cancer, there are currently no biomarkers to guide selection of therapeutic options. This prospective biomarker trial evaluated the feasibility and potential clinical utility of circulating tumor DNA (ctDNA) analysis to inform adjuvant therapy decision making.
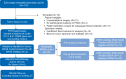
Materials and methods: Patients considered by the multidisciplinary team to have resectable pancreatic adenocarcinoma were enrolled. Pre- and post-operative samples for ctDNA analysis were collected. PCR-based-SafeSeqS assays were used to identify mutations at codon 12, 13 and 61 of KRAS in the primary pancreatic tumor and to detect ctDNA. Results of ctDNA analysis were correlated with CA19-9, recurrence-free and overall survival (OS). Patient management was per standard of care, blinded to ctDNA data.
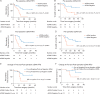
Results: Of 112 patients consented pre-operatively, 81 (72%) underwent resection. KRAS mutations were identified in 91% (38/42) of available tumor samples. Of available plasma samples (N = 42), KRAS mutated ctDNA was detected in 62% (23/37) pre-operative and 37% (13/35) post-operative cases. At a median follow-up of 38.4 months, ctDNA detection in the pre-operative setting was associated with inferior recurrence-free survival (RFS) [hazard ratio (HR) 4.1; P = 0.002)] and OS (HR 4.1; P = 0.015). Detectable ctDNA following curative intent resection was associated with inferior RFS (HR 5.4; P < 0.0001) and OS (HR 4.0; P = 0.003). Recurrence occurred in 13/13 (100%) patients with detectable ctDNA post-operatively, including in seven that received gemcitabine-based adjuvant chemotherapy.
Conclusion: ctDNA studies in localized pancreatic cancer are challenging, with a substantial number of patients not able to undergo resection, not having sufficient tumor tissue for analysis or not completing per protocol sample collection. ctDNA analysis, pre- and/or post-surgery, is a promising prognostic marker. Studies of ctDNA guided therapy are justified, including of treatment intensification strategies for patients with detectable ctDNA post-operatively who appear at very high risk of recurrence despite gemcitabine-based adjuvant therapy.
Keywords: adjuvant therapy; biomarkers; circulating tumor DNA; liquid biopsy; pancreatic cancer; pancreatic ductal adenocarcinoma.
© The Author(s) 2019. Published by Oxford University Press on behalf of the European Society for Medical Oncology. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures
Comment in
-
ctDNA to detect minimal residual disease in pancreatic cancer: moving into clinical trials.Ann Oncol. 2019 Sep 1;30(9):1410-1413. doi: 10.1093/annonc/mdz236. Ann Oncol. 2019. PMID: 31418008 No abstract available.
Similar articles
-
Mutant KRAS Circulating Tumor DNA Is an Accurate Tool for Pancreatic Cancer Monitoring.Oncologist. 2018 May;23(5):566-572. doi: 10.1634/theoncologist.2017-0467. Epub 2018 Jan 25. Oncologist. 2018. PMID: 29371474 Free PMC article.
-
Circulating tumor DNA status and dynamics predict recurrence in patients with resected extrahepatic cholangiocarcinoma.J Hepatol. 2025 May;82(5):861-870. doi: 10.1016/j.jhep.2024.10.043. Epub 2024 Nov 10. J Hepatol. 2025. PMID: 39532185 Clinical Trial.
-
Circulating Tumor DNA Analyses as Markers of Recurrence Risk and Benefit of Adjuvant Therapy for Stage III Colon Cancer.JAMA Oncol. 2019 Dec 1;5(12):1710-1717. doi: 10.1001/jamaoncol.2019.3616. JAMA Oncol. 2019. PMID: 31621801 Free PMC article. Clinical Trial.
-
A Review of Circulating Tumor DNA (ctDNA) in Pancreatic Cancer: Ready for the Clinic?J Gastrointest Cancer. 2025 Jan 21;56(1):50. doi: 10.1007/s12029-024-01151-2. J Gastrointest Cancer. 2025. PMID: 39836305 Review.
-
Circulating tumor DNA as a prognostic indicator in resectable pancreatic ductal adenocarcinoma: A systematic review and meta-analysis.Sci Rep. 2019 Nov 18;9(1):16971. doi: 10.1038/s41598-019-53271-6. Sci Rep. 2019. PMID: 31740696 Free PMC article.
Cited by
-
Building on the clinical applicability of ctDNA analysis in non-metastatic pancreatic ductal adenocarcinoma.Sci Rep. 2024 Jul 13;14(1):16203. doi: 10.1038/s41598-024-67235-y. Sci Rep. 2024. PMID: 39003322 Free PMC article.
-
Study protocol for a prospective, open-label, single-arm, phase II study on the combination of tislelizumab, nab-paclitaxel, gemcitabine, and concurrent radiotherapy as the induction therapy for patients with locally advanced and borderline resectable pancreatic cancer.Front Oncol. 2022 Aug 18;12:879661. doi: 10.3389/fonc.2022.879661. eCollection 2022. Front Oncol. 2022. PMID: 36059628 Free PMC article.
-
Clinical applications of circulating tumor-derived DNA in the management of gastrointestinal cancers - current evidence and future directions.Front Oncol. 2022 Sep 29;12:970242. doi: 10.3389/fonc.2022.970242. eCollection 2022. Front Oncol. 2022. PMID: 36248993 Free PMC article. Review.
-
Conversion Surgery for Advanced Pancreatic Cancer.J Clin Med. 2019 Nov 12;8(11):1945. doi: 10.3390/jcm8111945. J Clin Med. 2019. PMID: 31718103 Free PMC article. Review.
-
Emodin sensitizes human pancreatic cancer cells to EGFR inhibitor through suppressing Stat3 signaling pathway.Cancer Manag Res. 2019 Sep 17;11:8463-8473. doi: 10.2147/CMAR.S221877. eCollection 2019. Cancer Manag Res. 2019. PMID: 31572001 Free PMC article.
References
-
- Australian Institute of Health and Welfare (AIHW). Australian Cancer Incidence and Mortality (ACIM) books Canberra: AIHW.
-
- Conroy T, Hammel P, Hebbar M. et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med 2018; 379(25): 2395–2406. - PubMed
-
- Neopotelomos JPP, Palmer DH, Ghaneh P. et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised phase 3 trial. Lancet 2017; 389(10073): 1011–1024. - PubMed
-
- Update-on-ABRAXANE-Combination-Therapy-in-the-Treatment-of-Metastatic-Triple-Negative-Breast-Cancer-and-Pancreatic-Cancer. 2019; https://ir.celgene.com/ (12 March 2019, date last accessed).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

