Plasma expanders for people with cirrhosis and large ascites treated with abdominal paracentesis
- PMID: 31251387
- PMCID: PMC6598734
- DOI: 10.1002/14651858.CD004039.pub2
Plasma expanders for people with cirrhosis and large ascites treated with abdominal paracentesis
Abstract
Background: Plasma volume expanders are used in connection to paracentesis in people with cirrhosis to prevent reduction of effective plasma volume, which may trigger deleterious effect on haemodynamic balance, and increase morbidity and mortality. Albumin is considered the standard product against which no plasma expansion or other plasma expanders, e.g. other colloids (polygeline , dextrans, hydroxyethyl starch solutions, fresh frozen plasma), intravenous infusion of ascitic fluid, crystalloids, or mannitol have been compared. However, the benefits and harms of these plasma expanders are not fully clear.
Objectives: To assess the benefits and harms of any plasma volume expanders such as albumin, other colloids (polygeline, dextrans, hydroxyethyl starch solutions, fresh frozen plasma), intravenous infusion of ascitic fluid, crystalloids, or mannitol versus no plasma volume expander or versus another plasma volume expander for paracentesis in people with cirrhosis and large ascites.
Search methods: We searched the Cochrane Hepato-Biliary Group Controlled Trials Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, LILACS, CNKI, VIP, Wanfang, Science Citation Index Expanded, and Conference Proceedings Citation Index until January 2019. Furthermore, we searched FDA, EMA, WHO (last search January 2019), www.clinicaltrials.gov/, and www.controlled-trials.com/ for ongoing trials.
Selection criteria: Randomised clinical trials, no matter their design or year of publication, publication status, and language, assessing the use of any type of plasma expander versus placebo, no intervention, or a different plasma expander in connection with paracentesis for ascites in people with cirrhosis. We considered quasi-randomised, retrieved with the searches for randomised clinical trials only, for reports on harms.
Data collection and analysis: We used standard methodological procedures expected by Cochrane. We calculated the risk ratio (RR) or mean difference (MD) using the fixed-effect model and the random-effects model meta-analyses, based on the intention-to-treat principle, whenever possible. If the fixed-effect and random-effects models showed different results, then we made our conclusions based on the analysis with the highest P value (the more conservative result). We assessed risks of bias of the individual trials using predefined bias risk domains. We assessed the certainty of the evidence at an outcome level, using GRADE, and constructed 'Summary of Findings' tables for seven of our review outcomes.
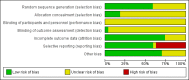
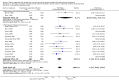
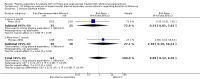
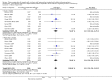
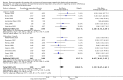
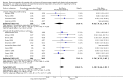
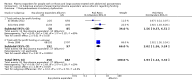
Main results: We identified 27 randomised clinical trials for inclusion in this review (24 published as full-text articles and 3 as abstracts). Five of the trials, with 271 participants, assessed plasma expanders (albumin in four trials and ascitic fluid in one trial) versus no plasma expander. The remaining 22 trials, with 1321 participants, assessed one type of plasma expander, i.e. dextran, hydroxyethyl starch, polygeline, intravenous infusion of ascitic fluid, crystalloids, or mannitol versus another type of plasma expander, i.e. albumin in 20 of these trials and polygeline in one trial. Twenty-five trials provided data for quantitative meta-analysis. According to the Child-Pugh classification, most participants were at an intermediate to advanced stage of liver disease in the absence of hepatocellular carcinoma, recent gastrointestinal bleeding, infections, and hepatic encephalopathy. All trials were assessed as at overall high risk of bias. Ten trials seemed not to have been funded by industry; twelve trials were considered unclear about funding; and five trials were considered funded by industry or a for-profit institution.We found no evidence of a difference in effect between plasma expansion versus no plasma expansion on mortality (RR 0.52, 95% CI 0.06 to 4.83; 248 participants; 4 trials; very low certainty); renal impairment (RR 0.32, 95% CI 0.02 to 5.88; 181 participants; 4 trials; very low certainty); other liver-related complications (RR 1.61, 95% CI 0.79 to 3.27; 248 participants; 4 trials; very low certainty); and non-serious adverse events (RR 1.04, 95% CI 0.32 to 3.40; 158 participants; 3 trials; very low certainty). Two of the trials stated that no serious adverse events occurred while the remaining trials did not report on this outcome. No trial reported data on health-related quality of life.We found no evidence of a difference in effect between experimental plasma expanders versus albumin on mortality (RR 1.03, 95% CI 0.82 to 1.30; 1014 participants; 14 trials; very low certainty); serious adverse events (RR 0.89, 95% CI 0.10 to 8.30; 118 participants; 2 trials; very low certainty); renal impairment (RR 1.17, 95% CI 0.71 to 1.91; 1107 participants; 17 trials; very low certainty); other liver-related complications (RR 1.10, 95% CI 0.82 to 1.48; 1083 participants; 16 trials; very low certainty); and non-serious adverse events (RR 1.37, 95% CI 0.66 to 2.85; 977 participants; 14 trials; very low certainty). We found no data on heath-related quality of life and refractory ascites.
Authors' conclusions: Our systematic review and meta-analysis did not find any benefits or harms of plasma expanders versus no plasma expander or of one plasma expander such as polygeline, dextrans, hydroxyethyl starch, intravenous infusion of ascitic fluid, crystalloids, or mannitol versus albumin on primary or secondary outcomes. The data originated from few, small, mostly short-term trials at high risks of systematic errors (bias) and high risks of random errors (play of chance). GRADE assessments concluded that the evidence was of very low certainty. Therefore, we can neither demonstrate or discard any benefit of plasma expansion versus no plasma expansion, and differences between one plasma expander versus another plasma expander.Larger trials at low risks of bias are needed to assess the role of plasma expanders in connection with paracentesis. Such trials should be conducted according to the SPIRIT guidelines and reported according to the CONSORT guidelines.
Conflict of interest statement
RGS: nothing to declare GP: nothing to declare CG: nothing to declare DN: nothing to declare GB: nothing to declare
Figures





























































Update of
- doi: 10.1002/14651858.CD004039
Similar articles
-
Radix Sophorae flavescentis versus no intervention or placebo for chronic hepatitis B.Cochrane Database Syst Rev. 2019 Apr 3;4(4):CD013089. doi: 10.1002/14651858.CD013089.pub2. Cochrane Database Syst Rev. 2019. PMID: 30941748 Free PMC article.
-
Acupuncture for chronic hepatitis B.Cochrane Database Syst Rev. 2019 Aug 22;8(8):CD013107. doi: 10.1002/14651858.CD013107.pub2. Cochrane Database Syst Rev. 2019. PMID: 31436846 Free PMC article.
-
Colloids versus crystalloids for fluid resuscitation in critically ill people.Cochrane Database Syst Rev. 2018 Aug 3;8(8):CD000567. doi: 10.1002/14651858.CD000567.pub7. Cochrane Database Syst Rev. 2018. PMID: 30073665 Free PMC article.
-
Glucocorticosteroids for people with alcoholic hepatitis.Cochrane Database Syst Rev. 2019 Apr 9;4(4):CD001511. doi: 10.1002/14651858.CD001511.pub4. Cochrane Database Syst Rev. 2019. PMID: 30964545 Free PMC article.
-
Acetyl-L-carnitine for patients with hepatic encephalopathy.Cochrane Database Syst Rev. 2019 Jan 5;1(1):CD011451. doi: 10.1002/14651858.CD011451.pub2. Cochrane Database Syst Rev. 2019. PMID: 30610762 Free PMC article.
Cited by
-
European Society of Intensive Care Medicine clinical practice guideline on fluid therapy in adult critically ill patients. Part 1: the choice of resuscitation fluids.Intensive Care Med. 2024 Jun;50(6):813-831. doi: 10.1007/s00134-024-07369-9. Epub 2024 May 21. Intensive Care Med. 2024. PMID: 38771364
-
Albumin Administration is Efficacious in the Management of Patients with Cirrhosis: A Systematic Review of the Literature.Hepat Med. 2020 Oct 27;12:153-172. doi: 10.2147/HMER.S264231. eCollection 2020. Hepat Med. 2020. PMID: 33149707 Free PMC article. Review.
-
Use of Human Albumin Administration for the Prevention and Treatment of Hyponatremia in Patients with Liver Cirrhosis: A Systematic Review and Meta-Analysis.J Clin Med. 2022 Oct 8;11(19):5928. doi: 10.3390/jcm11195928. J Clin Med. 2022. PMID: 36233795 Free PMC article.
-
Treatment for ascites in adults with decompensated liver cirrhosis: a network meta-analysis.Cochrane Database Syst Rev. 2020 Jan 16;1(1):CD013123. doi: 10.1002/14651858.CD013123.pub2. Cochrane Database Syst Rev. 2020. PMID: 31978257 Free PMC article.
-
Refractory Ascites in Patients With Cirrhosis.JGH Open. 2025 Jul 31;9(8):e70245. doi: 10.1002/jgh3.70245. eCollection 2025 Aug. JGH Open. 2025. PMID: 40756101 Free PMC article. No abstract available.
References
References to studies included in this review
Abdel‐Khalek 2010 {published data only}
-
- Abdel‐Khalek EE, Arif S. Randomized trial comparing human albumin and hydroxyethyl starch 6% as plasma expanders for treatment of patients With liver cirrhosis and tense ascites after large‐volume paracentesis. Journal of Hepatology 2010;52:S327.
-
- Abdel‐Khalek EE, Arif SE. Randomized trial comparing human albumin and hydroxyethyl starch 6% as plasma expanders for treatment of patients with liver cirrhosis and tense ascites following large volume paracentesis. Arab Journal of Gastroenterology 2010;11:24‐9.
Al Sebaey 2012 {published data only}
-
- Al Sebaey A, Abdel‐Razek W, Bassuni A, Rewisha EA, Khalil M, Waked I, et al. Alternatives to albumin for prevention of paracentesis induced circulatory dysfunction (PICD) in cirrhosis. Hepatology 2012;56(S1):915A.
Altman 1998 {published data only}
-
- Altman C, Bernard B, Roulot D, Vitte R‐L, Ink O. Randomized comparative multicenter study of hydroxyethyl starch versus albumin as a plasma expander in cirrhotic patients with tense ascites treated with paracentesis. European Journal of Gastroenterology & Hepatology 1998;10:5‐10. [PUBMED: 9512946] - PubMed
Baik 2000 {published data only}
-
- Baik SK, Kim HS, Lee DK, Kim IH, Kwon SO. Hemodynamic changes after single large volume paracentesis (SLVP) in cirrhosis with tense ascites ‐ focusing on the effect of albumin as plasma expander. Korean Journal of Medicine 2000;58:276‐82.
Bertrán 1991 {published data only}
-
- Bertrán X, Fernández‐Bañares F, Planas F, Cabré E, Morillas R, León R, et al. Effect on the nutritional status of total paracentesis plus albumin or dextran‐70 i.v. infusion for the treatment of tense ascites in liver cirrhosis [Repercusión sobre el estado nutricional energético‐proteico de la paracentesis total asociada a la infusión de albúmina o dextano‐70 en el tratamiento de la ascitis a tensión en la cirrhosis hepática]. Revista Espanola de Enfermedades Digestivas 1991;79(5):320‐4. - PubMed
Bruno 1992 {published data only}
Degoricija 2003 {published data only}
-
- Degoricija V, Zjacic‐Rotkvic V, Marout J, Sefer S, Troskot B. Clinical and neurohumoral response to posture, physical exercise, and ascites treatment in Child‐Pugh C liver cirrhosis: randomized prospective trial. Croatian Medical Journal 2003;44(2):178‐86. [PUBMED: 12698509] - PubMed
-
- Degoricija V, Zjacic‐Rotkvic V, Troskot B, Šefer S. Postparacentesis neurohumoral changes and impairment of renal water excretion in Child‐Pugh C cirrhosis with and without volume replacement. Journal of Hepatology 2001;34:51.
Descos 1983 {published data only}
-
- Descos L, Gauthier A, Levy VG, Michel H, Quinton A, Rueff B, et al. Comparison of six treatments of ascites in patients with liver cirrhosis. A clinical trial. Hepato‐gastroenterology 1983;30:15‐20. [PUBMED: 6339343] - PubMed
Fassio 1992 {published data only}
-
- Fassio E, Terg R, Landeira G, Abecasis R, Salemne M, Podesta A, et al. Paracentesis with dextran 70 vs paracentesis with albumin in cirrhosis with tense ascites. Results of a randomized study. Journal of Hepatology 1992;14(2‐3):310‐6. [PUBMED: 1380024] - PubMed
García‐Compeán 1993 {published data only}
-
- García‐Compeán D, Villareal JZ, Cuevas HB, Cantú DAG, Estrella M, Tamez EG, et al. Total therapeutic paracentesis (TTP) with and without intravenous albumin in the treatment of cirrhotic tense ascites: a randomized controlled trial. Liver 1993;13(5):233‐8. [PUBMED: 8259034] - PubMed
García‐Compeán 2002 {published data only}
-
- García‐Compeán D, Blanc P, Larrey D, Daures J‐P, Hirtz J, Mendoza E, et al. Treatment of cirrhotic tense ascites with dextran‐40 versus albumin associated with large volume paracentesis. A randomized controlled trial. Annals of Hepatology 2002;1(1):29‐35. [PUBMED: 15114293] - PubMed
Ginès 1988 {published data only}
-
- Ginès P, Titó L, Arroyo V, Planas R, Panés J, Viver J, et al. Randomized comparative study of therapeutic paracentesis with and without intravenous albumin in cirrhosis. Gastroenterology 1988;94(6):1493‐1502. [PUBMED: 3360270] - PubMed
Ginès 1996 {published data only}
-
- Ginès A, Fernández‐Esparrach G, Monescillo A, Vila C, Domènech E, Abecasis R, et al. Randomized trial comparing albumin, dextran 70, and polygeline in cirrhotic patients with ascites treated by paracentesis. Gastroenterology 1996;111(4):1002‐10. [PUBMED: 8831595] - PubMed
-
- International Group for the Study of Ascites in Cirrhosis. Comparison of albumin, dextran‐70 and hemaccel in the prevention of effective hypovolemia in cirrhotic patients with ascites treated with total paracentesis. A randomized multicenter study. Hepatology 1995;22(4 Suppl):220A.
Graziotto 1997 {published data only}
-
- Graziotto A, Rossaro L, Inturri P, Salvagnini M. Reinfusion of concentrated ascitic fluid versus total paracentesis. A randomized prospective trial. Digestive Diseases and Sciences 1997;42(8):1708‐14. [PUBMED: 9286238] - PubMed
Hernández Pérez 1995 {published data only}
-
- Hernández Pérez RE, Aguilar Ramírez JR, Hernández Lopez JM, Gómez Maganda y Silva T. Total paracentesis and infusion of dextran 70 vs albumin in cirrhotic patients with tense ascites [Paracentesis masiva con reposición de dextran 70 vs albúmina en pacientes cirróticos con ascitis a tensión]. Revista de Gastroenterologia de Mexico 1995;60(1):22‐6. [PUBMED: 7543691] - PubMed
Kang 1998 {published data only}
-
- Kang KE, Kim BH, Dong SH, Kim HJ, Chang YW, Lee JII, et al. The efficacy of hydroxyethyl starch as plasma expander in cirrhotic patients with tense ascites treated with large volume paracentesis. Korean Journal of Gastroenterology 1998;31:651‐60.
Khan 2015 {published data only}
-
- Khan MU, Rahim IU, Latif M. Hemaccel as a cheaper alternative to human albumin for plasma expansion during paracentesis in cirrhotic patients. Pakistan Journal of Medical and Health Sciences 2015;9(3):948‐51.
Luca 1995 {published data only}
-
- Luca A, García‐Pagán J, Bosch J, Feu F, Jiménez W, Ginés A, et al. Beneficial effects of intravenous albumin infusion on the hemodynamic and humoral changes after total paracentesis. Hepatology 1995;22(3):753‐8. [PUBMED: 7657279] - PubMed
Mehta 1998 {published data only}
-
- Mehta NN, Abraham P, Ashar VJ, Shikare SS, Savant SS, Karnad DR, et al. Ascitic fluid filtration and intravenous infusion versus total‐volume paracentesis with infusion of plasma expander in cirrhosis with tense or intractable ascites. Indian Journal of Gastroenterology 1998;17(3):93‐6. - PubMed
Méndez 1991 {published data only}
-
- Méndez C, Soriano G, Guarner C, Tomás A, Moyano D, Toro H, et al. Total paracentesis in cirrhotics with refractory ascites: albumin vs HES as plasmatic expanders. Journal of Hepatology 1991;13(Suppl 2):S145.
Moreau 2006 {published data only}
-
- Moreau R, Valla D‐C, Durand‐Zaleski I, Bronowicki J‐P, Durand F, Chaput J‐C, et al. Comparison of outcome in patients with cirrhosis and ascites following treatment with albumin or a synthetic colloid: a randomised controlled pilot trial. Liver International 2006;26(1):46‐54. [PUBMED: 16420509] - PubMed
Planas 1990 {published data only}
-
- Planas R, Ginès P, Arroyo V, Llach J, Panés J, Vargas V, et al. Dextran‐70 versus albumin as plasma expanders in cirrhotic patients with tense ascites treated with total paracentesis. Results of a randomized study. Gastroenterology 1990;99(6):1736‐44. [PUBMED: 1699835] - PubMed
-
- Planas R, Llach J, Ginès P, Panés J, Vargas V, Salmeron JM, et al. Albumin versus dextran‐70 in cirrhotics with tense ascites treated with total paracentesis. Final results of a multicenter randomized comparative study. Journal of Hepatology 1990;11(Suppl 2):S49. - PubMed
Salerno 1991 {published data only}
-
- Salerno F, Badalamenti S, Lorenzano E, Moser P, Incerti P. Randomized comparative study of hemaccel vs albumin infusion after total paracentesis in cirrhotic patients with refractory ascites. Hepatology 1991;13(4):707‐13. [PUBMED: 1826281] - PubMed
Simonetti 1991 {published data only}
-
- Rinaldi F, Simonetti RG, Maisano S, Dino O, Milazzo G, Luca A, et al. Refractory ascites in cirrhosis: treatment with total paracentesis and reinfusion of filtrated and concentrated ascitic fluid. Italian Journal of Gastroenterology 1990;22(4):261.
-
- Simonetti RG, Rinaldi F, Milazzo G, Dino O, Maisano S, Luca A, et al. Treatment of refractory ascites: a randomized prospective trial of total paracentesis with concentrated ascitic fluid reinfusion vs repeated paracentesis and albumin infusion. Italian Journal of Gastroenterology 1991;23(7):473.
Smart 1990 {published data only}
-
- Smart HL, Triger DR. A randomised prospective trial comparing daily paracentesis and intravenous albumin with recirculation in diuretic refractory ascites. Journal of Hepatology 1990;10(2):191‐7. [PUBMED: 2185298] - PubMed
Sola‐Vera 2003 {published data only}
-
- Sola‐Vera J, Minana J, Soriano G, Gonzalez B, Planella M, Lopez‐Balaguer JM, et al. Expansion with saline vs albumin in cirrhotic patients with tense ascites treated with total paracentesis. Result of a prospective randomized study. Gastroenterologia y Hepatologia 2001;24(2):90 A6.
-
- Sola‐Vera J, Miñana J, Ricart E, Planella M, González B, Torras X, et al. Randomized trial comparing albumin and saline in the prevention of paracentesis‐induced circultory dysfunction in cirrhotic patients with ascites. Hepatology 2003;37(5):1147‐53. [PUBMED: 12717396] - PubMed
Zhao 2000 {published data only}
-
- Zhao J, Yuan C, Wang D, LI X. Mannitolum infusion on cirrhotic patients with tense ascites treated by paracentesis. Chinese Medical Journal 2000;113(1):27‐30. [PUBMED: 11775204] - PubMed
References to studies excluded from this review
Antillon 1990 {published data only}
-
- Antillon MR, Runyon BA, Steindel H, Montano AA. Is albumin infusion needed after large volume paracentesis in patients with refractory ascites?. Gastroenterology 1990;98:A565.
Antillon 1991 {published data only}
-
- Antillon MR, Runyon BA. Postparacentesis plasma expansion prevents asymptomatic laboratory abnormalities, but does it have any impact on morbidity or mortality?. Gastroenterology 1991;101(5):1455‐7. [PUBMED: 1936822] - PubMed
Nasr 2010 {published data only}
Zaak 2001 {published data only}
-
- Zaak D, Paquet KJ, Kuhn R. Prospective study comparing human albumin vs reinfusion of ultrafiltated‐ascitic fluid after total paracentesis in cirrhotic patients with tense ascites. Zeitschrift fur Gastroenterologie 2001;39(1):5‐10. [PUBMED: 11215366] - PubMed
References to ongoing studies
EudraCT 2010‐019783‐37 {unpublished data only}
-
- 2010‐019783‐37/CZ. Two doses of albumin after large paracentesis in cirrhotics with refractory ascites: a randomized study. clinicaltrialsregister.eu/ctr‐search/trial/2010‐019783‐37/CZ (first received 15 June 2010).
NCT03202524 {unpublished data only}
-
- NCT03202524. Fresh frozen plasma as a substitute for albumin in patients receiving a large volume paracentesis. clinicaltrials.gov/ct2/show/study/NCT03202524 (first received 28 June 2017).
Additional references
AASLD 2009
-
- Runyon BA, AASLD Practice Guidelines Committee. Management of adult patients with ascites due to cirrhosis: an update. Hepatology 2009;49(6):2087‐107. [PUBMED: 19475696] - PubMed
AASLD 2012
-
- Runyon BA, AASLD. AASLD Practice Guideline. Management of adult patients with ascites due to cirrhosis: update 2012. Hepatology 2013;57(4):1651‐3. [PUBMED: 23463403] - PubMed
AIFA 2018
-
- Agenzia Italiana del Farmaco. Nota informativa importante sulla restrizione d’uso di HES. www.agenziafarmaco.gov.it/sites/default/files/IT_DHPC_HES_common.pdf (accessed prior to 16 June 2019).
AISF 2016
-
- Italian Association for the Study of the Liver (AISF), Italian Society of Transfusion Medicine and Immunohaematology (SIMTI). AISF‐SIMTI position paper: the appropriate use of albumin in patients with liver cirrhosis. Digestive and Liver Disease 2016;48(1):4‐15. [PUBMED: 26802734] - PubMed
Al Sebaey 2013
-
- Al Sebaey A, Razek WA, Bassuni A, Khalil M, Rewisha E, Waked I. Low‐dose albumin is equally effective as standard dose albumin in preventing PICD following large volume paracentesis (LVP). Hepatology International 2013;7 (Suppl 1):S451.
Alderson 2002
-
- Alderson P, Bunn F, Lefebvre C, Li Wan Po A, Li L, Roberts I, et al. the Albumin Reviewers. Human albumin solution for resuscitation and volume expansion in critically ill patients. Cochrane Database of Systematic Reviews 2002, Issue 1. [DOI: 10.1002/14651858.CD001208; PUBMED: 11869596] - DOI - PubMed
Alessandria 2011
-
- Alessandria C, Elia C, Mazzabotta L, Risso A, Andrealli A, Spandra M, et al. Prevention of paracentesis‐induced circulatory dysfunction in cirrhosis: standard vs half albumin doses. A prospective, randomized, unblinded pilot study. Digestive and Liver Disease 2011;43(11):881‐6. [PUBMED: 21741331] - PubMed
Antillon 1993
-
- Antillon MR, Runyon BA. Extracorporeal ultrafiltration and intravenous reinfusion of ascitic fluid vs. large‐volume paracentesis: a randomised controlled trial. Hepatology 1993;17(3):520‐2. [PUBMED: 8444427] - PubMed
Arora 2018a
-
- Arora V, Maiwall R, Thomas SS, Rajan V, Ali R, Kumar G, et al. Albumin decreases the incidence of paracentesis induced circulatory dysfunction with less than 5 litres of ascitic tap in acute on chronic liver failure (ACLF) patients: randomized controlled trial (NCT02467348). Journal of Hepatology 2018;68(S1):S42‐S43.
Arora 2018b
-
- Arora V, Maiwall R, Ali R, Kumar G, Thomas SS, Jain P, et al. Paracentesis induced circulatory dysfunction with modest paracentesis is reduced by albumin in acute‐on‐chronic liver failure: a randomized controlled trial (NCT02467348). Indian Journal of Gastroenterology 2018;37(S1):A17.
Arroyo 1996
-
- Arroyo V, Ginès P, Gerbes AL, Dudley FJ, Gentilini P, Laffi G, et al. International Ascites Club. Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. Hepatology 1996;23(1):164‐76. [PUBMED: 8550036] - PubMed
Arroyo 2016
-
- Arroyo V, Moreau R, Kamath PS, Jalan R, Ginès P, Nevens F, et al. Acute‐on‐chronic liver failure in cirrhosis. Nature Reviews. Disease Primers 2016;2:16041. [PUBMED: 27277335] - PubMed
Bajaj 2018
-
- Bajaj JS, Tandon P, O'Leary JG, Biggins SW, Wong F, Kamath PS, et al. The impact of albumin use on resolution of hyponatremia in hospitalized patients with cirrhosis. American Journal of Gastroenterology 2018;113(9):1339. [PUBMED: 29880972] - PubMed
Balshem 2011
-
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. [PUBMED: 21208779] - PubMed
Bapat 1998
-
- Bapat PP, Raine GJ. Fluid resuscitation with colloid or crystalloid solutions. Use of dextran‐70 for fluid resuscitation has been dying out. BMJ 1998;317(7153):278. [PUBMED: 9729073] - PubMed
Beale 1998
-
- Beale RJ, Wyncoll DL, McLuckie A. Human albumin administration in critically ill patients. Analysis is superficial and conclusions exaggerated. BMJ 1998;317(7162):884. [PUBMED: 9786698] - PubMed
Begg 1994
-
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50(4):1088‐101. [PUBMED: 7786990] - PubMed
Bernardi 2012
-
- Bernardi M, Caraceni P, Navickis RJ, Wilkes MM. Albumin infusion in patients undergoing large‐volume paracentesis: a meta‐analysis of randomized trials. Hepatology 2012;55(4):1172‐81. [PUBMED: 22095893] - PubMed
Bernardi 2014
-
- Bernardi M, Caraceni P, Navickis RJ, Wilkes MM. Reply to PMID 22095893. Hepatology 2014;60(4):1443‐5. [PUBMED: 24585539] - PubMed
Bernardi 2018
-
- Bernardi M, Caraceni P. Novel perspectives in the management of decompensated cirrhosis. Nature Reviews. Gastroenterology and Hepatology 2018;15(12):753‐64. [PUBMED: 30026556] - PubMed
Bernardi 2019
-
- Bernardi M, Zaccherini G, Caraceni P. Pro: the role of albumin in pre‐liver transplant management. Liver Transplantation 2019;25(1):128‐34. [PUBMED: 30346096] - PubMed
Biggins 2006
-
- Biggins SW, Kim WR, Terrault NA, Saab S, Balan V, Schiano T, et al. Evidence‐based incorporation of serum sodium concentration into MELD. Gastroenterology 2006;130(6):1652‐60. [PUBMED: 16697729] - PubMed
Boyer 2016
-
- Boyer TD, Sanyal AJ, Wong F, Frederick RT, Lake JR, O'Leary JG, et al. Terlipressin plus albumin Is more effective than albumin alone in improving renal function in patients with cirrhosis and hepatorenal syndrome type 1. Gastroenterology 2016;150(7):1579‐89.e2. [PUBMED: 26896734] - PubMed
Brok 2008
-
- Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta‐analyses. Journal of Clinical Epidemiology 2008;61(8):763‐9. [PUBMED: 18411040] - PubMed
Brok 2009
-
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta‐analyses may be inconclusive ‐ trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta‐analyses. International Journal of Epidemiology 2009;38(1):287‐98. [PUBMED: 18824466] - PubMed
Caraceni 2018
-
- Caraceni P, Riggio O, Angeli P, Alessandria C, Neri S, Foschi FG, et al. Long‐term albumin administration in decompensated cirrhosis (ANSWER): an open‐label randomised trial. Lancet 2018;391(10138):2417‐29. [PUBMED: 29861076] - PubMed
Castellini 2018
Chalmers 1998
-
- Chalmers I. Human albumin administration in critically ill patients. I would not want an albumin transfusion. BMJ 1998;317(7162):885. [PUBMED: 9786701] - PubMed
Chen 2014
-
- Chen RP, Chen C, Yu JY, Huang XL, Zhou MT, Huang ZM. Trial sequence meta‐analysis can reject false‐positive result calculated from conventional meta‐analysis. Hepatology 2014;60(4):1442‐3. [PUBMED: 24585570] - PubMed
Cochrane Injuries Group Albumin Reviewers 1998
Corder 1998
-
- Corder AP. Fluid resuscitation with colloid or crystalloid solutions. Virtually identical article had appeared in Cochrane Library. BMJ 1998;317(7153):279. [PUBMED: 9729075] - PubMed
D'Amico 1986
-
- D'Amico G, Morabito A, Pagliaro L, Marubini E. Survival and prognostic indicators in compensated and decompensated cirrhosis. Digestive Diseases and Sciences 1986;31(5):468‐75. [PUBMED: 3009109] - PubMed
D'Amico 2006
-
- D’Amico G, Garcia‐Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. Journal of Hepatology 2006;44(1):217‐31. [PUBMED: 16298014] - PubMed
Di Pascoli 2019
-
- Pascoli M, Fasolato S, Piano S, Bolognesi M, Angeli P. Long‐term administration of human albumin improves survival in patients with cirrhosis and refractory ascites. Liver International 2019;39(1):98‐105. [PUBMED: 30230204] - PubMed
EASL 2016
-
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Liver transplantation. Journal of Hepatology 2016;64(2):433‐85. [PUBMED: 26597456] - PubMed
EASL 2018
-
- European Association for the Study of the Liver. EASL clinical practice guidelines for the management of patients with decompensated cirrhosis. Journal of Hepatology 2018;69(2):406‐60. [PUBMED: 29653741] - PubMed
Egger 1997
EMA 2018
-
- European Medicines Agency. Hydroxyethyl starch solutions: CMDh introduces new measures to protect patients. www.ema.europa.eu/documents/referral/hydroxyethyl‐starch‐article‐107i‐re... (accessed prior to 16 June 2019).
FDA 2013
-
- Food, Drug Administration. FDA safety communication: boxed warning on increased mortality and severe renal injury, and additional warning on risk of bleeding, for use of hydroxyethylstarch solutions in some settings. fffenterprises.com/assets/downloads/Article‐FDASafetyCommunicationBoxedW... (accessed prior to 16 June 2019).
Fisher 1922
-
- Fisher RA. On the interpretation of X2 from contingency tables, and the calculation of P. Journal of the Royal Statistical Society 1922;85(1):87‐94.
Frame 1998
-
- Frame JD, Moiemem N. Human albumin administration in critically ill patients. Statisticians not trained in burns care should not evaluate data. BMJ 1998;317(7162):884‐5. [PUBMED: 9786700] - PubMed
Garcia‐Martinez 2013
-
- Garcia‐Martinez R, Caraceni P, Bernardi M, Gines P, Arroyo V, Jalan R. Albumin: pathophysiologic basis of its role in the treatment of cirrhosis and its complications. Hepatology 2013;58(5):1836‐46. [PUBMED: 23423799] - PubMed
Garcia‐Tsao 2018
-
- Garcia‐Tsao G. Long‐term albumin in cirrhosis: is it the ANSWER?. Lancet 2018;391(10138):2391‐2. [PUBMED: 29861077] - PubMed
Gartlehner 2018
-
- Gartlehner G, Nussbaumer‐Streit B, Wagner G, Patel S, Swinson‐Evans T, Dobrescu A, et al. Increased risks for random errors are common in outcomes graded as high certainty of evidence. Journal of Clinical Epidemiology 2018;106:50‐9. [DOI: 10.1016/j.jclinepi.2018.10.009; PUBMED: 30342970] - DOI - PubMed
Gentilini 1999
-
- Gentilini P, Casini‐Raggi V, Fiore G, Romanelli RG, Buzzelli G, Pinzani M, et al. Albumin improves the response to diuretics in patients with cirrhosis and ascites: results of a randomized, controlled trial. Journal of Hepatology 1999;30(4):639‐45. [PUBMED: 10207805] - PubMed
Ginés 1987
-
- Ginés P, Arroyo V, Quintero E, Planas R, Bory F, Cabrera J, et al. Comparison of paracentesis and diuretics in the treatment of cirrhotics with tense ascites. Results of a randomized study. Gastroenterology 1987;93(2):234‐41. [PUBMED: 3297907] - PubMed
Ginés 2008
-
- Ginés P, Guevara M. Hyponatremia in cirrhosis: pathogenesis, clinical significance, and management. Hepatology 2008;48(3):1002‐10. [PUBMED: 18671303] - PubMed
Gluud 2007
-
- Gluud C, Brok J, Gong Y, Koretz RL. Hepatology may have problems with putative surrogate outcome measures. Journal of Hepatology 2007;46(4):734‐42. [PUBMED: 17316871] - PubMed
Gluud 2015
-
- Gluud C, Nikolova D, Klingenberg SL, Cochrane Hepato‐Biliary Group. About Cochrane (Cochrane Review Groups (CRGs)). CHBG Module. hbg.cochrane.org (accessed prior to 16 June 2019).
Goodman 1998
-
- Goodman NW. Human albumin administration in critically ill patients. Paper failed to mention earlier review. BMJ 1998;317(7162):884. [PUBMED: 9786699] - PubMed
Gosling 1998
-
- Gosling P. Fluid resuscitation with colloid or crystalloid solutions. Newer synthetic colloids should not be abandoned. BMJ 1998;317(7153):277. [PUBMED: 9729070] - PubMed
Guevara 2009
-
- Guevara M, Baccaro ME, Torre A, Gómez‐Ansón B, Ríos J, Torres F, et al. Hyponatremia is a risk factor of hepatic encephalopathy in patients with cirrhosis: a prospective study with time‐dependent analysis. American Journal of Gastroenterology 2009;104(6):1382‐9. [PUBMED: 19455124] - PubMed
Guevara 2010
-
- Guevara M, Baccaro ME, Ríos J, Martín‐Llahí M, Uriz J, Ruiz del Arbol L, et al. Risk factors for hepatic encephalopathy in patients with cirrhosis and refractory ascites: relevance of serum sodium concentration. Liver International 2010;30(8):1137‐42. [PUBMED: 20602681] - PubMed
Guevara 2012
-
- Guevara M, Terra C, Nazar A, Solà E, Fernández J, Pavesi M. Albumin for bacterial infections other than spontaneous bacterial peritonitis in cirrhosis. A randomized, controlled study. Journal of Hepatology 2012;57(4):759‐65. [PUBMED: 22732511] - PubMed
Guyatt 2008
Guyatt 2011a
-
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction ‐ GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [PUBMED: 21195583] - PubMed
Guyatt 2011b
-
- Guyatt GH, Oxman AD, Kunz R, Atkins D, Brozek J, Vist G, et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. Journal of Clinical Epidemiology 2011;64(4):395‐400. [PUBMED: 21194891] - PubMed
Guyatt 2011c
-
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso‐Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence ‐ study limitations (risk of bias). Journal of Clinical Epidemiology 2011;64(4):407‐15. [PUBMED: 21247734] - PubMed
Guyatt 2011d
-
- Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J, et al. GRADE guidelines: 5. Rating the quality of evidence ‐ publication bias. Journal of Clinical Epidemiology 2011;64(12):1277‐82. [PUBMED: 21802904] - PubMed
Guyatt 2011e
-
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence ‐ imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. [PUBMED: 21839614] - PubMed
Guyatt 2011f
-
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence ‐ inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294‐302. [PUBMED: 21803546] - PubMed
Guyatt 2011g
-
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence ‐ indirectness. Journal of Clinical Epidemiology 2011;64(12):1303‐10. [PUBMED: 21802903] - PubMed
Guyatt 2011h
-
- Guyatt GH, Oxman AD, Sultan S, Glasziou P, Akl EA, Alonso‐Coello P, et al. GRADE guidelines: 9. Rating up the quality of evidence. Journal of Clinical Epidemiology 2011;64(12):1311‐6. [PUBMED: 21802902] - PubMed
Guyatt 2013a
-
- Guyatt G, Oxman AD, Sultan S, Brozek J, Glasziou P, Alonso‐Coello P, et al. GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. Journal of Clinical Epidemiology 2013;66(2):151‐7. [PUBMED: 22542023] - PubMed
Guyatt 2013b
-
- Guyatt GH, Oxman AD, Santesso N, Helfand M, Vist G, Kunz R, et al. GRADE guidelines: 12. Preparing summary of findings tables ‐ binary outcomes. Journal of Clinical Epidemiology 2013;66(2):158‐72. [PUBMED: 22609141] - PubMed
Guyatt 2013c
-
- Guyatt GH, Thorlund K, Oxman AD, Walter SD, Patrick D, Furukawa TA, et al. GRADE guidelines: 13. Preparing summary of findings tables and evidence profiles ‐ continuous outcomes. Journal of Clinical Epidemiology 2013;66(2):173‐83. [PUBMED: 23116689] - PubMed
Haase 2013
Haase 2014
-
- Haase NR. Hydroxyethyl starch in sepsis. Danish Medical Journal 2014;61(1):B4764. [PUBMED: 24393593] - PubMed
Harbord 2006
-
- Harbord RM, Egger M, Sterne JA. A modified test for small‐study effects in meta‐analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25(20):3443‐57. [PUBMED: 16345038] - PubMed
Hartog 2014
He 2015
Higgins 2002
-
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [PUBMED: 12111919] - PubMed
Higgins 2003
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. available from handbook.cochrane.org.
Hollis 1999
ICH 1997
-
- International Conference on Harmonisation Expert Working Group. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH Harmonised Tripartite Guideline. Guideline for Good Clinical Practice CFR & ICH Guidelines. Vol. 1, Philadelphia (PA) 19063‐2043, USA: Barnett International/PAREXEL, 1997.
ICTRP 2011
-
- World Health Organization. International Clinical Trials Registry Platform (ICTRP). www.who.int/ictrp/en/ (accessed prior to 16 June 2016).
Jakobsen 2014
Jakobsen 2017
Jakobsen 2018
-
- Jakobsen JC, Nielsen EE, Koretz RL, Gluud C. Do direct acting antivirals cure chronic hepatitis C?. BMJ 2018;361:K1382. [PUBMED: 29748173] - PubMed
Jalan 2007
-
- Jalan R, Mookerjee RP, Cheshire LM, Williams R, Davies NA. Albumin infusion for severe hyponatremia in patients with refractory ascites: a randomized clinical trial. Journal of Hepatology 2007;46(1 Suppl):S95. [PUBMED: 17571340] - PubMed
Kaag 1998
-
- Kaag M, Zoetmulder FA. Human albumin administration in critically ill patients. More research into proper use of albumin is needed. BMJ 1998;317(7162):883. [PUBMED: 9786696] - PubMed
Kim 2008
Kjaergard 2001
-
- Kjaergard LL, Villumsen J, Gluud C. Reported methodological quality and dicrepancies between small and large randomized trials in meta‐analysis. Annals of Internal Medicine 2001;135(11):982‐9. [PUBMED: 11730399] - PubMed
Kwok 2013
-
- Kwok CS, Krupa L, Mahtani A, Kaye D, Rushbrook SM, Phillips MG, et al. Albumin reduces paracentesis‐induced circulatory dysfunction and reduces death and renal impairment among patients with cirrhosis and infection: a systematic review and meta‐analysis. Biomed Research International 2013;2013:1‐8. [DOI: 10.1155/2013/295153; PUBMED: 24222902] - DOI - PMC - PubMed
Kütting 2017
-
- Kütting F, Schubert J, Franklin J, Bowe A, Hoffmann V, Demir M, et al. Insufficient evidence of benefit regarding mortality due to albumin substitution in HCC‐free cirrhotic patients undergoing large volume paracentesis. Journal of Gastroenterology and Hepatology 2017;32(2):327–38. [PUBMED: 27149296] - PubMed
Landini 1985
-
- Landini S, Coli U, Fracasso A, Morachiello P, Righetto F, Scanferla F, et al. Spontaneous ascites filtration and reinfusion (SAFR) in cirrhotic patients. International Journal of Artificial Organs 1985;8(5):227‐80. [PUBMED: 4086118] - PubMed
Lawler 1998
-
- Lawler PG, Morgan GA. Human albumin administration in critically ill patients. Modified editorial might have restrained media response. BMJ 1998;317(7162):885. [PUBMED: 9786702] - PubMed
Lewis 2018
Lundh 2017
Makin 1998
-
- Makin A, Plenderleith L. Fluid resuscitation with colloid or crystalloid solutions. One conclusion could be that hypertonic saline is better than colloids in trauma. BMJ. 1998;317(7153):277‐8. [PUBMED: 9729071] - PubMed
Martín‐Llahí 2008
-
- Martín‐Llahí M, Pépin MN, Guevara M, Díaz F, Torre A, Monescillo A, et al. Terlipressin and albumin vs albumin in patients with cirrhosis and hepatorenal syndrome: a randomized study. Gastroenterology 2008;134(5):1352‐9. [PUBMED: 18471512] - PubMed
McAnulty 1998
-
- McAnulty GR, Grounds RM. Fluid resuscitation with colloid or crystalloid solutions. Eight studies should have been excluded. BMJ 1998;317(7153):278. [PUBMED: 9729072] - PubMed
McCormick 1990
Mohanty 2015
Moher 1998
-
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. [PUBMED: 9746022] - PubMed
Moore 2003
-
- Moore KP, Wong F, Gines P, Bernardi M, Ochs A, Salerno F, et al. The management of ascites in cirrhosis: report on the consensus conference of the International Ascites Club. Hepatology 2003;38(1):258‐66. [PUBMED: 12830009] - PubMed
Mustafa 2013
-
- Mustafa RA, Santesso N, Brozek J, Akl EA, Walter SD, Norman G, et al. The GRADE approach is reproducible in assessing the quality of evidence of quantitative evidence syntheses. Journal of Clinical Epidemiology 2013;66(7):736‐42. [PUBMED: 23623694] - PubMed
Nadel 1998
-
- Nadel S, Marriage S, Munter C, Britto J, Habibi P, Levin M. Human albumin administration in critically ill patients. Review did not provide recommendations for alternative treatment. BMJ 1998;317(7162):882‐3. [PUBMED: 9786694] - PubMed
Nel 1998
-
- Nel R. Human albumin administration in critically ill patients. Critical analysis of original studies has to take place. BMJ 1998;317(7162):882. [PUBMED: 9786693] - PubMed
O'Brien 2019
-
- O'Brien A. Con: the unclear benefit of albumin. Liver Transplantation 2019;25(1):135‐9. [PUBMED: 30447173] - PubMed
Offringa 1998
O’Brien 2018
Perel 2013
Perner 2014
-
- Perner A, Haase N, Winkel P, Guttormsen AB, Tenhunen J, Klemenzson G, et al. Long‐term outcomes in patients with severe sepsis randomised to resuscitation with hydroxyethyl starch 130/0.42 or Ringer's acetate. Intensive Care Medicine 2014;40(7):927‐34. [PUBMED: 24807084] - PubMed
Petros 1998
Pugh 1973
-
- Pugh RN, Murray‐Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. British Journal of Surgery 1973;60(8):646‐9. [PUBMED: 4541913] - PubMed
Qureshi 2016
-
- Qureshi SH, Rizvi SI, Patel NN, Murphy GJ. Meta‐analysis of colloids versus crystalloids in critically ill, trauma and surgical patients. British Journal of Surgery 2016;103(1):14‐26. [PUBMED: 26522616] - PubMed
RevMan 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Riordan 1998
-
- Riordan FA, Williams A, Thomson AP. Human albumin administration in critically ill patients. Albumin has been used in meningococcal disease. BMJ 317;7162:883. [PUBMED: 9786695] - PubMed
Roberts 1998
-
- Roberts I. Human albumin administration in critically ill patients. Authors’ response. BMJ 1998;317(7162):886. - PubMed
Roberts 1999
Roberts 2011
Romanelli 2006
Ruiz‐del‐Arbol 1997
-
- Ruiz‐del–Arbol L, Monescillo A, Jimenén W, Garcia‐Plaza A, Arroyo V, Rodés J. Paracentesis‐induced circulatory dysfunction: mechanism and effect on hepatic hemodynamics in cirrhosis. Gastroenterology 1997;113(2):579‐86. [PUBMED: 9247479] - PubMed
Runyon 1998
-
- Runyon BA. Management of adult patients with ascites caused by cirrhosis. Hepatology 1998;27(1):264‐72. [PUBMED: 9425946] - PubMed
Rücker 2008
-
- Rücker G, Schwarzer G, Carpenter J. Arcsine test for publication bias in meta‐analyses with binary outcomes. Statistics in Medicine 2008;27(5):746‐63. [PUBMED: 17592831] - PubMed
Salerno 2013
-
- Salerno F, Navickis RJ, Wilkes MM. Albumin infusion improves outcomes of patients with spontaneous bacterial peritonitis: a meta‐analysis of randomized trials. Clinical Gastroenterology and Hepatology 2013;11(2):123‐30.e1. [PUBMED: 23178229] - PubMed
Saló 1997
-
- Saló J, Ginès A, Ginès P, Piera C, Jiménez W, Guevara M, et al. Effect of therapeutic paracentesis on plasma volume and transvascular escape rate of albumin in patients with cirrhosis. Journal of Hepatology 1997;27(4):645‐53. [PUBMED: 9365040] - PubMed
Savović 2012a
-
- Savović J, Jones HE, Altman D, Harris R, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials: combined analysis of meta‐epidemiological studies. Health Technology Assessment 2012;16(35):1‐82. [PUBMED: 22989478] - PubMed
Savović 2012b
-
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Annals of Internal Medicine 2012;157(6):429‐38. [PUBMED: 22945832] - PubMed
Schierhout 1998a
Schierhout 1998b
-
- Schierhout GH, Roberts I. Authors’ reply. BMJ 1998;317(7153):279.
Schulz 1995
-
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [PUBMED: 7823387] - PubMed
Shwe 1998
-
- Shwe KH, Bhavnani M. Human albumin administration in critically ill patients. Some patients may benefit. BMJ 1998;317(7162):885‐6. [PUBMED: 9786703] - PubMed
Sola 2012
-
- Solà E, Watson H, Graupera I, Turón F, Barreto R, Rodríguez E, et al. Factors related to quality of life in patients with cirrhosis and ascites: relevance of serum sodium concentration and leg edema. Journal of Hepatology 2012;57(6):1199‐206. [PUBMED: 22824819] - PubMed
Solà 2018
-
- Solà E, Solé C, Simón‐Talero M, Martín‐Llahí M, Castellote J, Garcia‐Martínez R, et al. Midodrine and albumin for prevention of complications in patients with cirrhosis awaiting liver transplantation. A randomized placebo‐controlled trial. Journal of Hepatology 2018;69(6):1250‐9. [PUBMED: 30138685] - PubMed
Soni 1998
-
- Soni N. Human albumin administration in critically ill patients. Validity of review methods must be assessed. BMJ 1998;317(7162):883‐4. [PUBMED: 9786697] - PubMed
Sort 1999
-
- Sort P, Navasa M, Arroyo V, Aldeguer X, Planas R, Ruiz‐del‐Arbol L, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. New England Journal of Medicine 1999;341(6):403‐9. [PUBMED: 10432325] - PubMed
Storebø 2018
-
- Storebø OJ, Pedersen N, Ramstad E, Kielsholm ML, Nielsen SS, Krogh HB, et al. Methylphenidate for attention deficit hyperactivity disorder (ADHD) in children and adolescents – assessment of adverse events in non‐randomised studies. Cochrane Database of Systematic Reviews 2018, Issue 5. [DOI: 10.1002/14651858.CD012069.pub2] - DOI - PMC - PubMed
Student 1908
-
- Student. The probable error of a mean. Biometrika 1908;6(1):1‐25.
Thorlund 2010
Thorlund 2011
-
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for Trial Sequential Analysis (TSA). www.ctu.dk/tsa/files/TSA_manual.pdf (accessed prior to 16 June 2019).
Thévenot 2015
-
- Thévenot T, Bureau C, Oberti F, Anty R, Louvet A, Plessier A, et al. Effect of albumin in cirrhotic patients with infection other than spontaneous bacterial peritonitis. A randomized trial. Journal of Hepatology 2015;62(4):822‐30. [PUBMED: 25463545] - PubMed
Titó 1990
-
- Titó L, Ginès P, Arroyo V, Planas R, Planés J, Rimola A, et al. Total paracentesis associated with intravenous albumin management of patients with cirrhosis and ascites. Gastroenterology 1990;98(1):146‐51. [PUBMED: 2293573] - PubMed
TSA 2011 [Computer program]
-
- Copenhagen Trial Unit, Center for Clinical Intervention Research, Rigshospitalet. TSA ‐ Trial Sequential Analysis. Version 0.9.5.10 Beta. Copenhagen, Denmark: Copenhagen Trial Unit, Center for Clinical Intervention Research, Rigshospitalet, 2011.
Vila 1998
-
- Vila MC, Solà R, Molina L, Andreu M, Coll S, Gana J, et al. Hemodynamic changes in patients developing effective hypovolemia after total paracentesis. Journal of Hepatology 1998;28(4):639‐45. [PUBMED: 9566833] - PubMed
Wang 2015
-
- Wang Y‐C, Feng M‐l. A meta‐analysis of albumin infusion in patients undergoing large‐volume paracentesis [大量放腹水后输注人体白蛋白疗效的 Meta分析]. Chinese Hepatology 2015;20(5):381‐5. [DOI: 10.14000/j.cnki.issn.1008-1704.2015.05.010] - DOI
Watts 1998
Wetterslev 2008
-
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. [PUBMED: 18083463] - PubMed
Wetterslev 2009
Wetterslev 2017
Wilkes 2001
-
- Wilkes M, Navickis RJ. Patient survival after human albumin administration. A meta‐analysis of randomized, controlled trials. Annals of Internal Medicine 2001;135(3):149‐64. [PUBMED: 11487482] - PubMed
Wong 2008
-
- Wong CL, Holroyd‐Leduc J, Thorpe KE, Straus SE. Does this patient have bacterial peritonitis or portal hypertension? How do I perform a paracentesis and analyze the results?. JAMA 2008;299(10):1166‐78. [PUBMED: 18334692] - PubMed
Wood 2008
Wyncoll 1998
-
- Wyncoll DL, Beale RJ, McLuckie A. Fluid resuscitation with colloid or crystalloid solutions. Conditions and patient groups were too heterogeneous to allow meaningful comparisons. BMJ 1998;317(7153):278‐9. [PUBMED: 9729074] - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

