Exogenous RNAi mechanisms contribute to transcriptome adaptation by phased siRNA clusters in Paramecium
- PMID: 31251800
- PMCID: PMC6735861
- DOI: 10.1093/nar/gkz553
Exogenous RNAi mechanisms contribute to transcriptome adaptation by phased siRNA clusters in Paramecium
Abstract
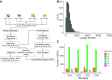
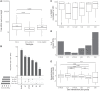
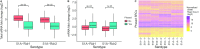
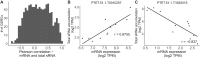
Extensive research has characterized distinct exogenous RNAi pathways interfering in gene expression during vegetative growth of the unicellular model ciliate Paramecium. However, role of RNAi in endogenous transcriptome regulation, and environmental adaptation is unknown. Here, we describe the first genome-wide profiling of endogenous sRNAs in context of different transcriptomic states (serotypes). We developed a pipeline to identify, and characterize 2602 siRNA producing clusters (SRCs). Our data show no evidence that SRCs produce miRNAs, and in contrast to other species, no preference for strand specificity of siRNAs. Interestingly, most SRCs overlap coding genes and a separate group show siRNA phasing along the entire open reading frame, suggesting that the mRNA transcript serves as a source for siRNAs. Integrative analysis of siRNA abundance and gene expression levels revealed surprisingly that mRNA and siRNA show negative as well as positive associations. Two RNA-dependent RNA Polymerase mutants, RDR1 and RDR2, show a drastic loss of siRNAs especially in phased SRCs accompanied with increased mRNA levels. Importantly, most SRCs depend on both RDRs, reminiscent to primary siRNAs in the RNAi against exogenous RNA, indicating mechanistic overlaps between exogenous and endogenous RNAi contributing to flexible transcriptome adaptation.
© The Author(s) 2019. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

